Application of octadecyl-modified polypeptide in preparing drugs for inhibiting platelet aggregation
A platelet aggregation and octadecyl technology, applied in the field of biomedicine, can solve the problems of increased bleeding risk, gastrointestinal irritation, weakened drug efficacy, etc., and achieve the effect of reducing bleeding risk.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention has no special limitation on the pharmaceutical dosage form and the preparation method of the dosage form, and the pharmaceutically acceptable dosage form and conventional preparation method of the polypeptide can be used for preparation. In the present invention, the polypeptide is the only active substance in the drug.
[0028] In the present invention, the sequence of the polypeptide is as follows:
[0029] SEQ ID No. 1: KEATSTF;
[0030] SEQ ID No.2 (Myristoylated-KafitidepT1):
[0031] Myristoylated-PLYKEA{pT}STFT;
[0032] SEQ ID No.3 (Myristoylated-KafitidepT2):
[0033] Myristoylated-PLYKEATS{pT}FT;
[0034] SEQ ID No.4 (Myristoylated-KafitidepT1-1-10AA): Myristoylated-PLYKEA{pT}STF;
[0035] SEQ ID No.5 (Myristoylated-Kafitide-6AA): Myristoylated-KEATST;
[0036] SEQ ID No.6 (Kafitidep-6AA): KEA{pT}ST;
[0037] SEQ ID No.7 (Kafitidep-51AA): KEA{pT}S;
[0038] SEQ ID No.8 (Kafitidep-5AA): EA{pT}ST;
[0039] SEQ ID No.9 (Kafitidep-4...
Embodiment 1
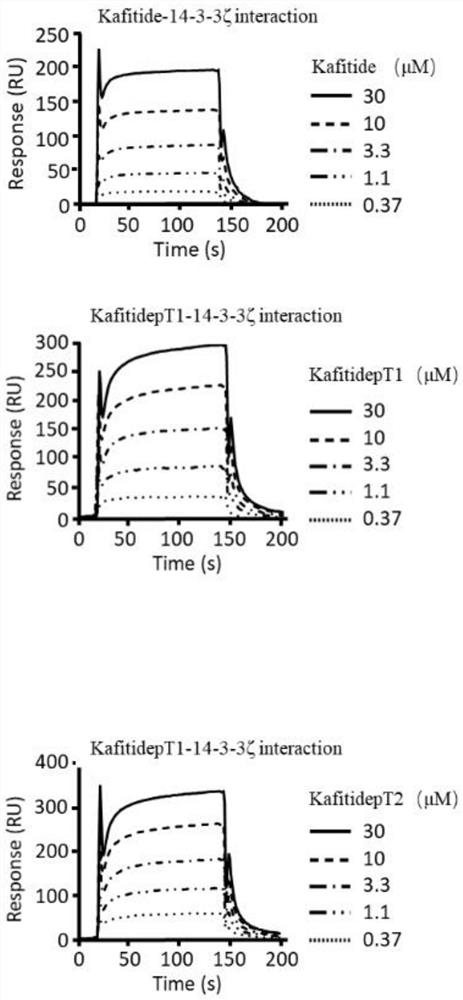
[0050] 1. Kafitide polypeptide (octadecylated polypeptide, hereinafter referred to as Kafitide polypeptide, amino acid sequence as shown in SEQ ID No.1) and phosphorylated analogs thereof (amino acid sequence as shown in SEQ ID No.2 and 3) and 14- 3-3ζ protein binding assay
[0051] The 14-3-3ζ protein (response value 9810RU) was bound to the CM5 chip (use 0.4M EDC (1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride) and 10mM NHS (N-Hydroxysuccinimide) to activate the chip, and use On 1M ethanolamine (ethanolamine) blocking), use the surface plasmon resonance instrument to the combination of different concentrations of Kafitide polypeptide and its phosphorylated analog (amino acid sequence shown in SEQ ID No.2 and 3) and 14-3-3ζ protein The conditions were detected, and the Kafitide polypeptide and its phosphorylated analogue (amino acid sequence shown in SEQ ID No.2 and 3) and 14-3-3ζ protein were obtained under different concentrations (0.37, 1.1, 3.3, 10, 30 μM res...
Embodiment 2
[0054] platelet aggregation inhibition test
[0055] platelet aggregation inhibition test
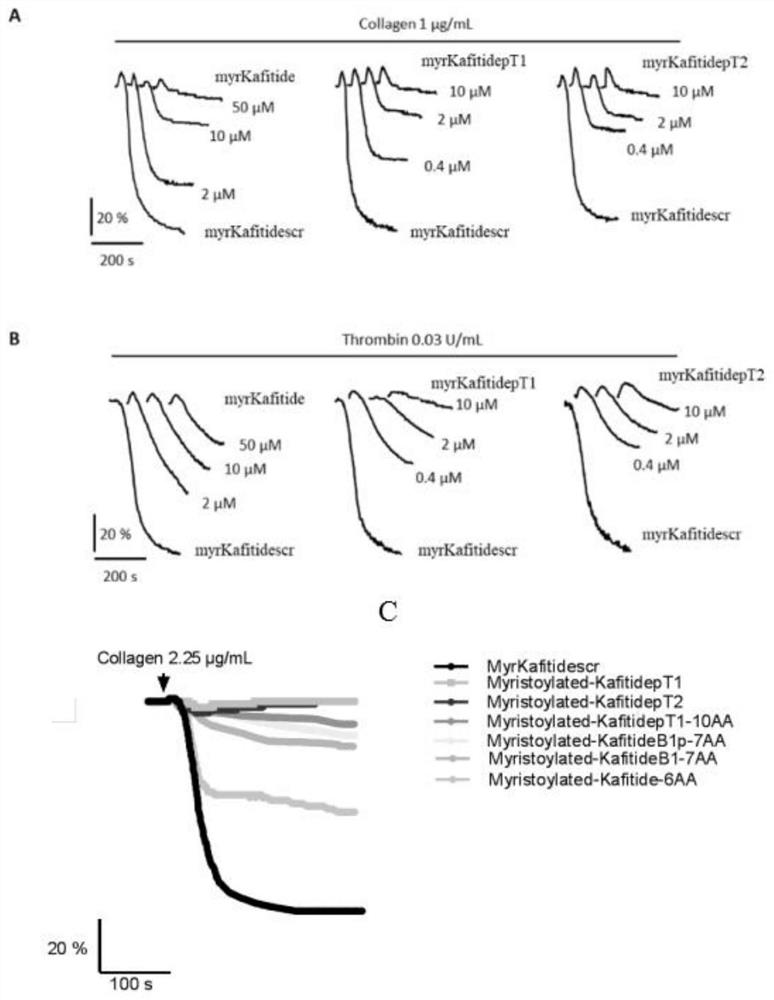
[0056] 1. After washing the platelets of healthy people with bench-top solution, dilute to 2.5×10 with bench-top solution B. 8 / mL. Take 300 μL of washed platelets, add samples of different concentrations, incubate at 37°C for 5 min, add 0.03 U / mL thrombin and 1 μg / mL collagen to induce aggregation, and draw the aggregation curve within 5 min on a platelet aggregometer. Platelet aggregation treated with the sample solvent was used as a control.
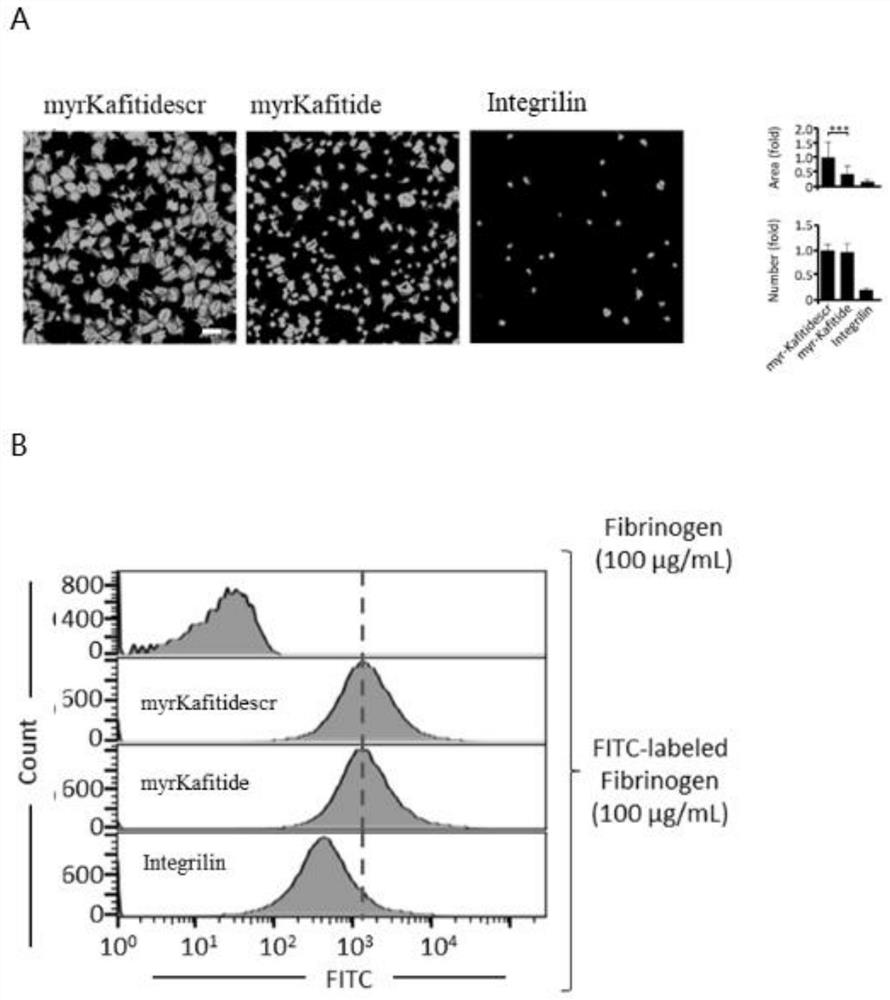
[0057] 2. If figure 2 As shown, Myristoylated-Kafitide polypeptide (amino acid sequence such as SEQ ID No.1) and its phosphorylated analogues (amino acid sequence such as SEQ ID No.2 and 3) inhibit the aggregation of platelets induced by Thrombin in a gradient-dependent manner. 10 μM Myristoylated-Kafitide polypeptide can inhibit the aggregation of about 50% platelets, and 1 μM Myristoylated-Kafitide polypeptide (amino acid sequence such a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap