Methods of reducing side effects of Anti-cd30 antibody drug conjugate therapy
A technology of antibody drugs and conjugates, applied in drug combinations, antibody medical components, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
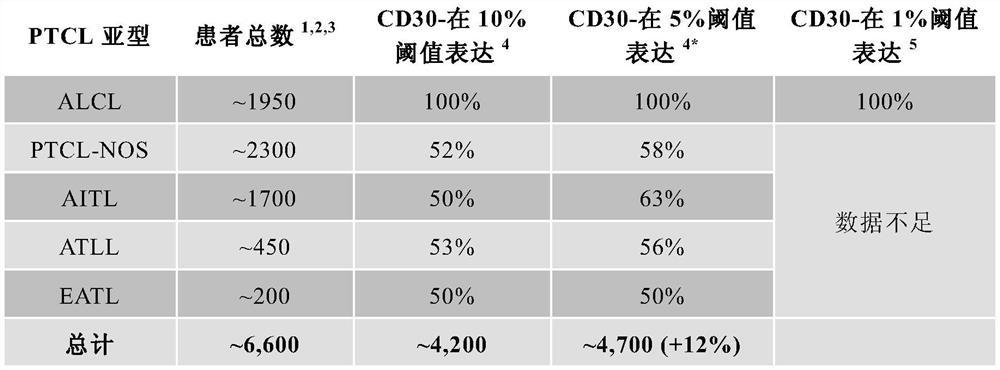
[0203] Berentuzumab vedotin was previously evaluated with sequential and concurrent multiagent chemotherapy in a phase 1 study of patients with newly diagnosed CD30-positive mature T-cell and NK-cell neoplasms, including sALCL (study SGN35-011) clinical safety and activity. A phase 1 study was conducted to determine the safety and activity of sequential and combined first-line treatment approaches of berentuzumab vedotin with CHOP or CHP chemotherapy. The maximum tolerated dose of berentuzumab vedotin administered concomitantly with CHP was 1.8 mg / kg. In an interim analysis of this study (data presented at T-cell Lymphoma Forum 2012), 20 patients in this study received berentuzumab vedotin 1.2 or 1.8 mg / kg in combination with CHP 6 cycles followed by vedotin every 3 weeks for up to 10 additional cycles in responding patients. The most common adverse events were nausea, fatigue, and peripheral sensory neuropathy. Among patients assessed for response after 6 cycles of berentu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com