Genetically engineered NK cell and preparation method and application thereof
A genetic engineering and NK cell technology, applied in the field of tumor immunotherapy, can solve the problems of not meeting the needs of critically ill patients, obtaining T cells is impractical, and obtaining T cells is cumbersome and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
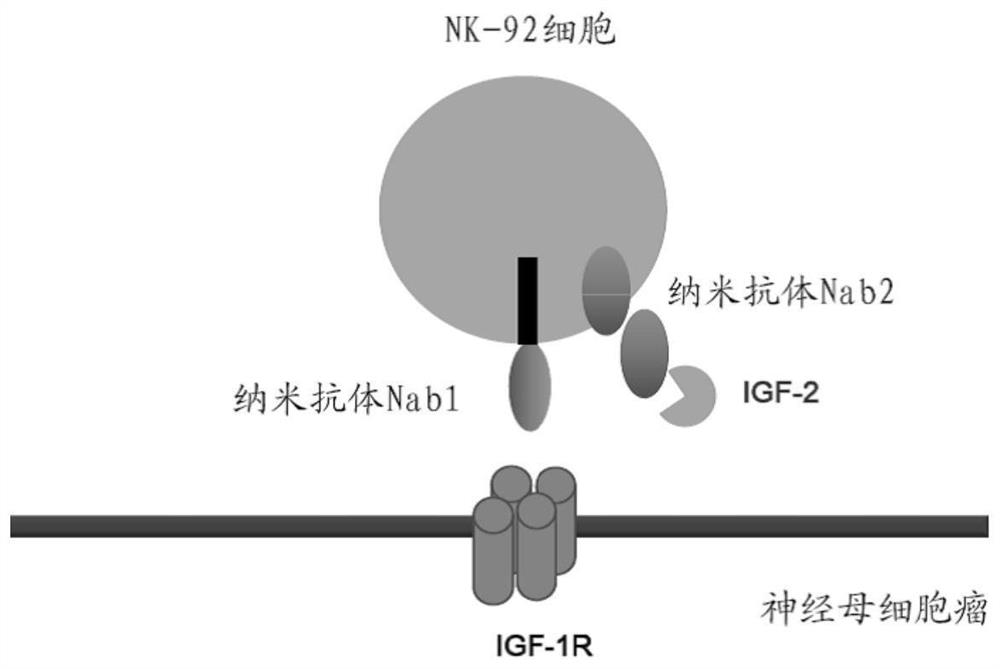
[0105] 1. Construction of CAR-engineered NK-92MI cells containing single nanobody Nab1
[0106] according to Figure 2A According to the design scheme shown in , by means of conventional techniques in the art (see, the "construction of the vector" part in the experimental method section), the vector is constructed, and the vector includes the transmembrane structure encoded by Nanobody Nab1 (NA-1), CD8 Nucleic acid of chimeric antigen receptor consisting of domain (TM), 4-1BB and CD3ζ.
[0107] For clarity, Figure 2A 5'LTR in means 5' long terminal repeat sequence; Ψ means viral packaging non-coding sequence; LS means coding sequence of leader sequence; NA-1 means coding sequence of Nanobody Nab1; CD8 TM means coding of CD8 transmembrane domain Sequences; 4-1BB and CD3ζ indicate the coding sequence of the intracellular signaling domain 4-1BB and CD3ζ, respectively; IRES indicates the coding sequence of the internal ribosome entry site; and ZsGreen1 indicates the coding sequ...
Embodiment 2
[0115] Example 2 was carried out using the same method as in Example 1, except that Figure 2B The schematic diagram of the design scheme of the vector used to construct the engineered NK cells comprising the bis-Nanobody of the present invention is shown in .
[0116] Figure 2B 2A in the scheme of design represents the coding sequence of Thoseaasigna virus 2A peptide; NA-2 represents the coding sequence of Nanobody Nab2.
[0117] Figure 3B A schematic diagram of the positive rate after sorting is shown in the plasmid containing the DNA sequence of the double nanobody CAR, transfected with lentivirus into NK-92MI cells. After sorting, the positive cell rate in the engineered CAR-NK-92MI cells was 68.1%. It was confirmed that engineered NK-92MI cells containing bis-Nanobodies Nab1 and Nab2 were obtained.
Embodiment 3
[0119] Example 3 was carried out using the same method as in Example 1, except that Figure 2C The design scheme for the construction of the vector used to construct the engineered NK-92MI cells comprising the single Nanobody Nab2 of the invention is shown in .
[0120] Next, the engineered NK-92MI cells obtained in Example 1, Example 2, and Example 3 above and the combination of Example 1 and Example 3 were tested through the evaluation example, that is, the engineered cell containing the single Nanobody Nab1 Cytotoxicity of NK-92MI cells and engineered NK-92MI cell combinations containing the single Nanobody Nab2.
[0121] Evaluation example
[0122] Calcein AM was used to perform cytotoxicity tests. First, LAN-1 cells were stained with calcein AM at 37 °C for 30 min, then the LAN-1 cells were washed with PBS, and the stained LAN-1 cells were prepared in this way. Since calcein AM can be converted to green fluorescent calcein inside the cells, after killing, dead LAN-1 ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com