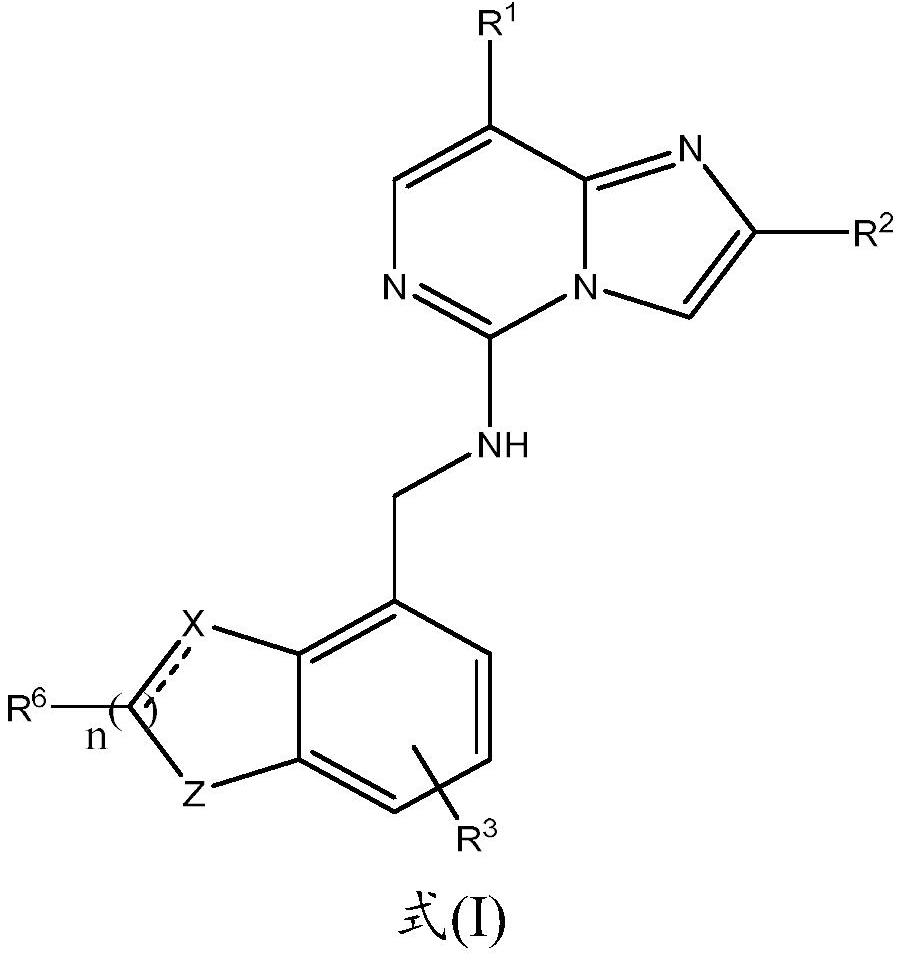
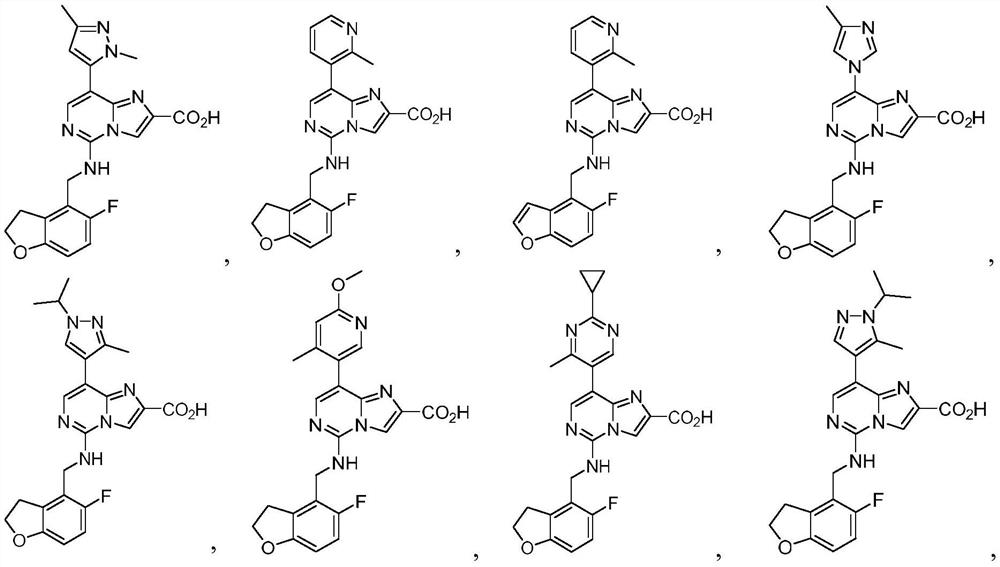
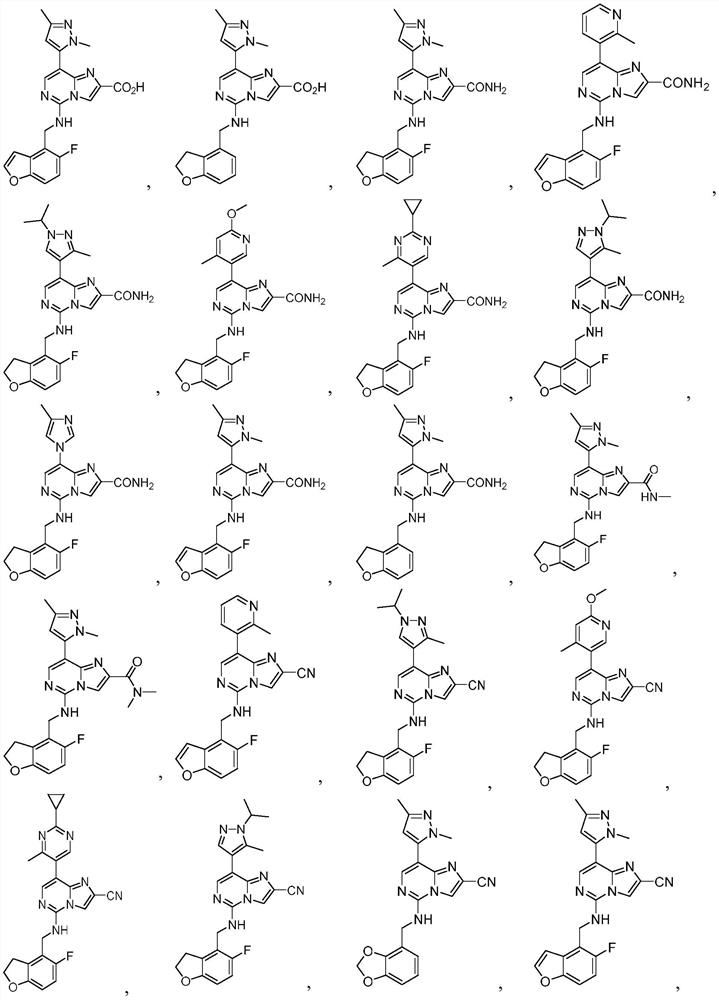
Prc2 inhibitors
A CR5, -COOR5 technology, applied in the field of compounds that inhibit polycomb protein inhibitory complex 2, can solve problems such as large recurrence risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0573] 8-(1,3-Dimethyl-1H-pyrazol-5-yl)-5-(((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)amino)imidazole And[1,2-c]pyrimidine-2-carboxylic acid
[0574]
[0575] 8-bromo-5-(((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)amino)imidazo[1,2-c]pyrimidine-2-carboxylic acid ethyl ester ( 0.100g, 230μmol, 1.00 equivalent), 1,3-dimethyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl ) pyrazole (81.6mg, 368μmol, 1.60 equivalent), sodium bicarbonate (77.2mg, 919μmol, 4.00 equivalent), Pd(dppf)Cl 2 (16.8 mg, 23.0 μmol, 0.100 equiv) in dioxane (2.10 mL) and water (0.700 mL) were purged three times with nitrogen. Subsequently, the mixture was stirred at 105 °C under nitrogen atmosphere for 1 h. The reaction mixture was filtered and concentrated in vacuo. The crude material was purified by prep-TLC (SiO 2 , PE:EA=2:3) to obtain 8-(1,3-dimethyl-1H-pyrazol-5-yl)-5-(((5-fluoro-2,3-dihydrobenzofuran -4-yl)methyl)amino)imidazo[1,2-c]pyrimidine-2-carboxylic acid ethyl ester (60.0 mg, 47.9% ...
Embodiment 2
[0579] 5-(((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)amino)-8-(2-methylpyridin-3-yl)imidazo[1,2-c ]pyrimidine-2-carboxylic acid
[0580]
[0581] To 8-bromo-5-(((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)amino)imidazo[1,2-c]pyrimidine-2-carboxylic acid ethyl ester ( 50.0mg, 115μmol, 1.00 equivalent), 2-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine ( 37.8 mg, 172 μmol, 1.50 eq) in dioxane (3.00 mL) were added water (1.00 mL) followed by Pd(dppf)Cl 2 (8.41 mg, 11.5 μmol, 0.100 equiv) and sodium bicarbonate (29.0 mg, 345 μmol, 3.00 equiv). The reaction mixture was stirred at 105 °C under nitrogen for 1 h. The mixture was cooled to 25 °C and filtered. The filtrate was concentrated in vacuo to provide a residue. The crude material was purified by prep-TLC (dichloromethane / methanol=10 / 1) to give 5-(((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)amino)-8 -(2-Methylpyridin-3-yl)imidazo[1,2-c]pyrimidine-2-carboxylic acid ethyl ester (40.0 mg, 75.0% yield, 96....
Embodiment 3
[0585] 5-(((5-fluorobenzofuran-4-yl)methyl)amino)-8-(2-methylpyridin-3-yl)imidazo[1,2-c]pyrimidine-2-carboxylic acid
[0586]
[0587] 8-Bromo-5-(((5-fluorobenzofuran-4-yl)methyl)amino)imidazo[1,2-c]pyrimidine-2-carboxylic acid ethyl ester (120 mg, 274 μmol, 1.00 equiv) , 2-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine (120 mg, 548 μmol, 2.00 equivalents), Sodium bicarbonate (69.0 mg, 822 μmol, 32.0 μL, 3.00 equiv) and Pd(dppf)Cl 2 (22.4 mg, 27.4 μmol, 0.100 equiv) in dioxane (3.00 mL) and water (0.600 mL) was purged with nitrogen and stirred at 105 °C under nitrogen atmosphere for 1 h. The mixture was concentrated under reduced pressure to obtain a residue. The crude material was purified by prep-TLC (DCM / methanol=20 / 1) to give 5-(((5-fluorobenzofuran-4-yl)methyl)amino)-8-(2-methylpyridine-3 -yl) Imidazo[1,2-c]pyrimidine-2-carboxylic acid ethyl ester (100 mg, 78.9% yield, 96.3% purity) as a brown solid. LCMS[M+1]: 446.2.
[0588]To 5-(((5-fluorobenzof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com