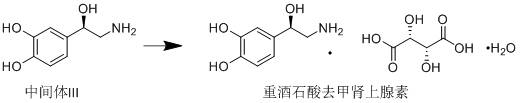
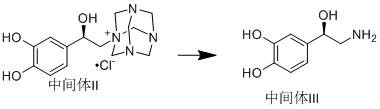
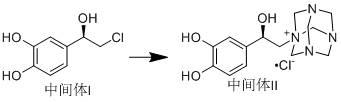
Method for asymmetrically synthesizing norepinephrine bitartrate
A technology of norepinephrine and bitartrate, which is applied in the field of norepinephrine bitartrate, can solve the problems of low resolution rate of racemic norepinephrine, increased cost and production cycle, and high risk, achieving dangerous Effect of reduction, reduction of cost increase, low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Under the protection of nitrogen, dissolve 2.43g (64.31mmol) of sodium borohydride in 30ml of tetrahydrofuran, cool to 10°C, add 7g (64.31mmol) of trimethylchlorosilane dropwise, after the addition is complete, heat up to 65°C and react for 4h, cool To 10℃-15℃, add 1g of (R)-(+)-alpha,alpha-diphenylprolinol, dissolve 10g (53.59mmol) of chloroacetylcatechol in 30ml of tetrahydrofuran at the same temperature, and slowly drip Add the above reaction system, after the dripping is completed, the temperature is raised to 25℃-30℃ and react for 24h. After the reaction is completed by TLC, 1N hydrochloric acid is added dropwise to adjust the pH to 1-2, after the tetrahydrofuran is evaporated, ammonia water is added dropwise to adjust the pH to 8- 9. Suction filtration, the filter cake was washed twice with water, the filter cake was collected, and dried under vacuum at 40°C-50°C to obtain 9.8 g of Intermediate I, with a yield of 96.95% and an ee value of 98%.
Embodiment 2
[0039]Under the protection of nitrogen, dissolve 3.47g (64.31mmol) of potassium borohydride in 30ml of tetrahydrofuran, cool to 10°C, add 12.12g (80.39mmol) of triethylchlorosilane dropwise, after the dropwise addition is complete, heat up to 55°C for 7h, Cool to 15℃-20℃, add 1.5g R)-(+)-alpha,alpha-dibenzylprolinol, and dissolve 10g (53.59mmol) of chloroacetylcatechol in 30ml tetrahydrofuran at the same temperature, slowly Add dropwise to the above reaction system. After the dropwise addition is completed, the temperature is raised to 25°C-30°C for 24 hours. After the reaction is completed by TLC, 1N hydrochloric acid is added dropwise to adjust the pH to 1-2, tetrahydrofuran is evaporated to dryness, and ammonia water is added dropwise to adjust the pH to 8 -9, suction filtration, the filter cake was washed twice with water, the filter cake was collected, and dried under vacuum at 40°C-50°C to obtain 8g of intermediate I with a yield of 79.14% and an ee value of 95.3%.
Embodiment 3
[0041]Under the protection of nitrogen, dissolve 3.04g (80.39mmol) of sodium borohydride in 30ml of tetrahydrofuran, cool to 10°C, add 12.40g (64.31mmol) of triisopropylchlorosilane dropwise, after the addition is complete, heat up to 55°C for 7h , Cool to 15℃-20℃, add 2g of R)-(+)-alpha,alpha-dinaphthylprolinol, dissolve 10g (53.59mmol) of chloroacetylcatechol in 30ml of tetrahydrofuran at the same temperature, slowly Add dropwise to the above reaction system. After the dropwise addition is completed, the temperature is raised to 25°C-30°C for 24 hours. After the reaction is completed by TLC, 1N hydrochloric acid is added dropwise to adjust the pH to 1-2, tetrahydrofuran is evaporated to dryness, and ammonia water is added dropwise to adjust the pH to 8 -9, suction filtration, the filter cake was washed twice with water, the filter cake was collected, and dried under vacuum at 40°C-50°C to obtain 8.6 g of Intermediate I with a yield of 85.08% and an ee value of 93.5%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap