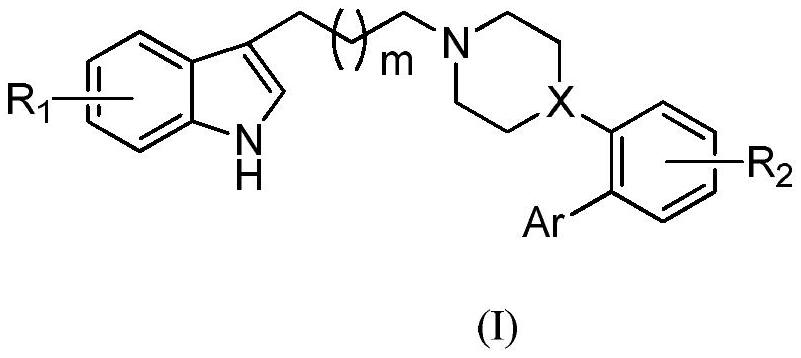
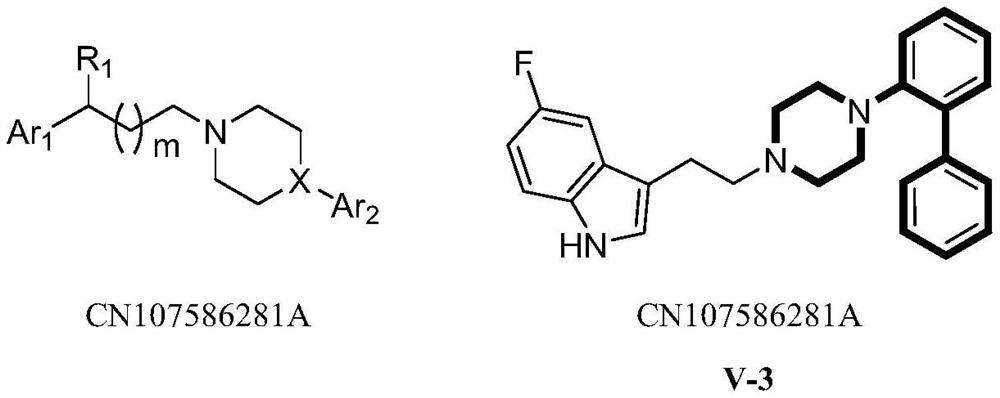
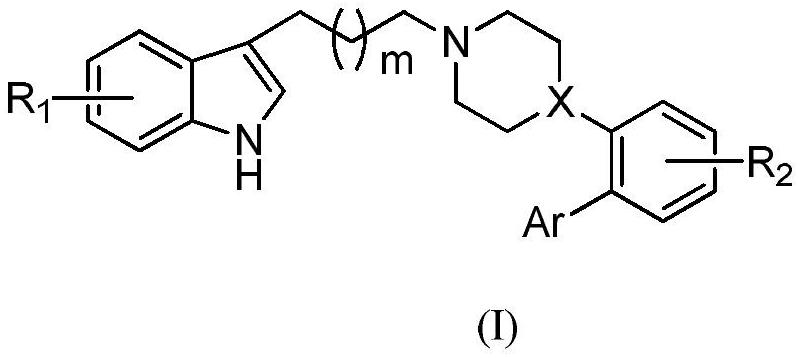
Application of Heterocyclic Substituted Phenylpiperazine or Phenylpiperidine Derivatives in Antidepressants
A technology of phenylpiperazine and phenylpiperidine is applied in the application field of heterocyclic substituted phenylpiperazine or phenylpiperidine derivatives in antidepressant drugs, and can solve the new antidepressant drug with three-target action Less problems, to achieve the effect of outstanding pharmacokinetic characteristics, outstanding targeting novelty, and remarkable novelty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] 5-Fluoro-3-(2-(4-(2-(pyridin-4-yl)phenyl)piperazin-1-yl)ethyl)-1H-indole (V-1) and its hydrochloride preparation of
[0124] o-iodobromobenzene compound (I) (0.1mol), N-Boc-piperazine (0.12mol), Pd 2 (dba) 3 (0.01mol) and Me 4 tBuXPhos (0.01 mol) was dissolved in toluene (300 mL), and sodium tert-butoxide (0.2 mol) was added, followed by general method 1 and step 1 to obtain intermediate II (white solid) with a yield of 72%. MS(m / z):341.08[M+1] + .
[0125] Intermediate (II) (0.05mol) was dissolved in methanol (300mL), and methanolic hydrochloride (4N, 0.2mol) was added. According to general method 1 and step 2, intermediate III (colorless liquid) was obtained with a yield of 80%. MS(m / z):241.03[M+1] + .
[0126] 2-(5-fluoro-1H-indol-3-yl)ethyl-4-methylbenzenesulfonate compound (IV) (0.05mol) and intermediate (III) (0.05mol) were dissolved in acetonitrile (200mL ), potassium carbonate (0.1 mol) was added. According to general method 1 and step 3, intermediate ...
Embodiment 2
[0130] 5-Fluoro-3-(2-(4-(2-(pyridin-3-yl)phenyl)piperazin-1-yl)ethyl)-1H-indole (V-2) and its hydrochloride preparation of
[0131] o-iodobromobenzene compound (I) (0.1mol), N-Boc-piperazine (0.12mol), Pd 2 (dba) 3 (0.01mol) and Me 4 tBuXPhos (0.01 mol) was dissolved in toluene (300 mL), and sodium tert-butoxide (0.2 mol) was added, followed by general method 1 and step 1 to obtain intermediate II (white solid) with a yield of 68%. MS(m / z):341.08[M+1] + .
[0132] Intermediate (II) (0.05mol) was dissolved in methanol (300mL), and methanolic hydrochloride (4N, 0.2mol) was added. According to general method 1 and step 2, intermediate III (colorless liquid) was obtained with a yield of 75%. MS(m / z):241.03[M+1] + .
[0133]2-(5-fluoro-1H-indol-3-yl)ethyl-4-methylbenzenesulfonate compound (IV) (0.05mol) and intermediate (III) (0.05mol) were dissolved in acetonitrile (200mL ), potassium carbonate (0.1 mol) was added. According to general method 1 and step 3, intermediate V...
Embodiment 3
[0137] 5-fluoro-3-(3-(4-(4-fluoro-2-(pyridin-3-yl)phenyl)piperazin-1-yl)propyl)-1H-indole (V-3) and Preparation of its hydrochloride
[0138] 2-Bromo-4-fluoro-1-iodobenzene compound (I) (0.1mol), N-Boc-piperazine (0.12mol), Pd 2 (dba) 3 (0.01mol) and Me 4 tBuXPhos (0.01 mol) was dissolved in toluene (300 mL), and sodium tert-butoxide (0.2 mol) was added, followed by general method 1 and step 1 to obtain intermediate II (white solid) with a yield of 70%. MS(m / z):359.10[M+1] + .
[0139] Intermediate (II) (0.05mol) was dissolved in methanol (300mL), and methanolic hydrochloride (4N, 0.2mol) was added. According to general method 1 and step 2, intermediate III (colorless liquid) was obtained with a yield of 78%. MS(m / z):259.12[M+1] + .
[0140] 3-(5-fluoro-1H-indol-3-yl)propyl-4-methylbenzenesulfonate compound (IV) (0.05mol) and intermediate (III) (0.05mol) were dissolved in acetonitrile (200mL ), potassium carbonate (0.1 mol) was added. According to general method 1 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com