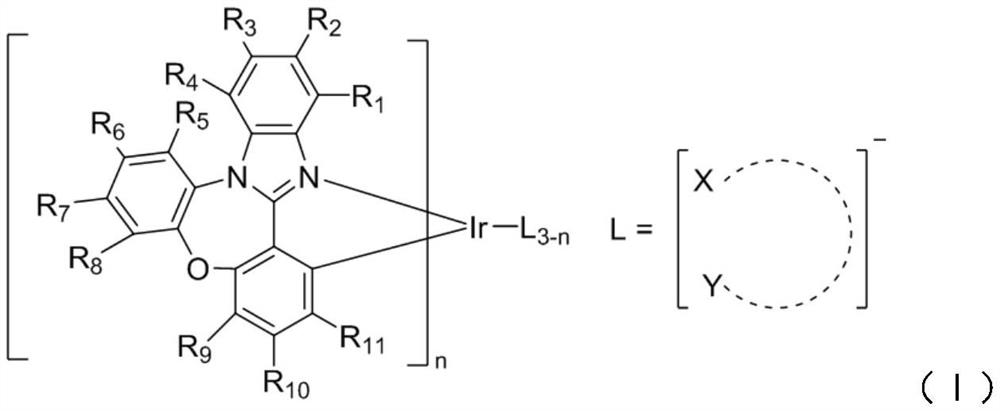
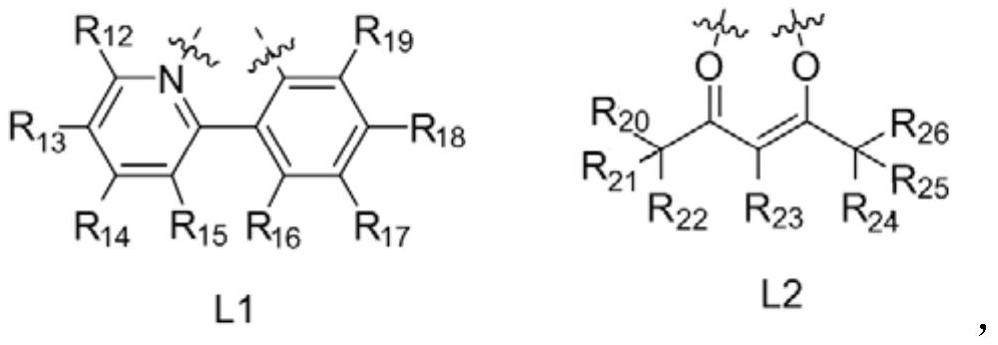
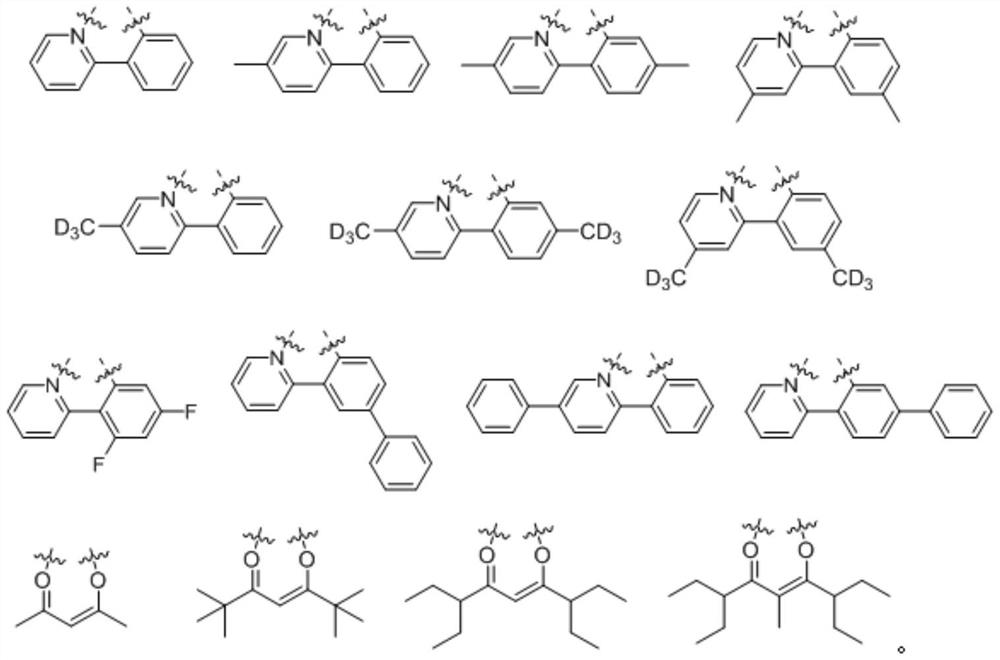
A kind of oxygen-containing organic electrophosphorescent material and its application
A phosphorescent material and electromechanical technology, applied in luminescent materials, organic chemistry, indium organic compounds, etc., can solve the problems of low phosphorescence efficiency, hindering commercialization, poor stability and lifespan, etc., to reduce purity and improve thermal stability sex, life extension effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Synthesis of Ligand P1
[0066]
[0067] The synthetic route is as follows:
[0068]
[0069] The specific experimental steps are:
[0070] (1) in the 2L there-necked flask equipped with mechanical stirring, add 2-fluoronitrobenzene (14.1g, 0.1mol), 2-bromoaniline (25.7g, 0.15mol), stir, argon protection, be warming up to 180 ℃, keep the reaction for more than 30 hours, during the reaction, the color gradually becomes red, and finally gradually becomes dark red. After the reaction, the organic phase was separated, extracted, dried, subjected to column chromatography, and the solvent was spin-dried to obtain 24.8 g of orange-red solid M1 with a yield of 85%.
[0071] (2) In a 1L three-necked flask equipped with mechanical stirring, add M1 (29.2g, 0.1mol), sodium sulfide nonahydrate (96g, 0.4mol), ethanol (200mL), water (100mL), nitrogen protection, heat to Reflux, the reaction was refluxed for 3 hours, and the reaction was terminated. The organic pha...
Embodiment 2
[0076] Example 2: Synthesis of Ligand P2
[0077]
[0078] With reference to the synthesis steps of Example 1, only in step (3), 2-bromo-4-methylbenzoyl chloride is used instead of o-bromobenzoyl chloride, and other raw materials and steps are the same as those in Example 1 to obtain ligand P2.
[0079] Product MS (m / e): 298.11; elemental analysis (C 20 H 14 N 2 O): theoretical value C: 80.52%, H: 4.73%, N: 9.39%; actual value C: 80.67%, H: 4.87%, N: 9.23%.
Embodiment 3
[0080] Example 3: Synthesis of Ligand P3
[0081]
[0082] Referring to the synthesis steps of Example 1, except that 2-bromo-4-methylaniline is used instead of 2-bromoaniline in step (1), other raw materials and steps are the same as those in Example 1, to obtain ligand P3.
[0083] Product MS (m / e): 298.11; elemental analysis (C 20 H 14 N 2 O): theoretical value C: 80.52%, H: 4.73%, N: 9.39%; actual value C: 80.67%, H: 4.85%, N: 9.21%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com