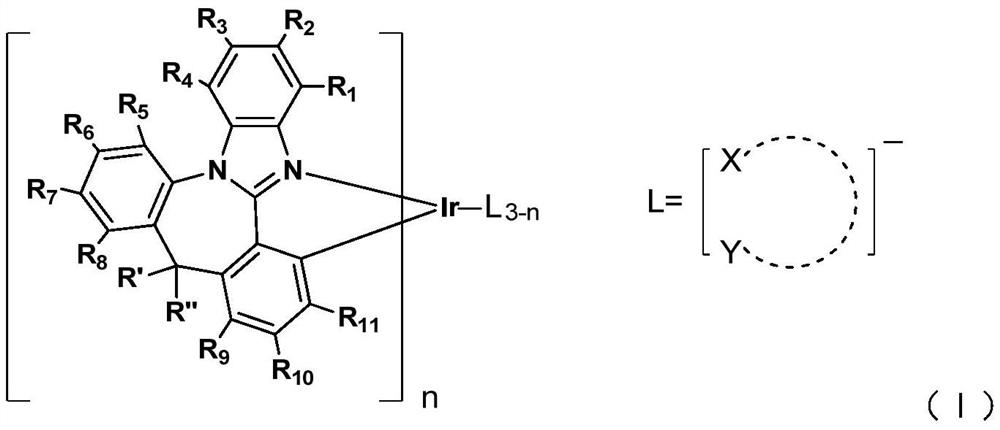
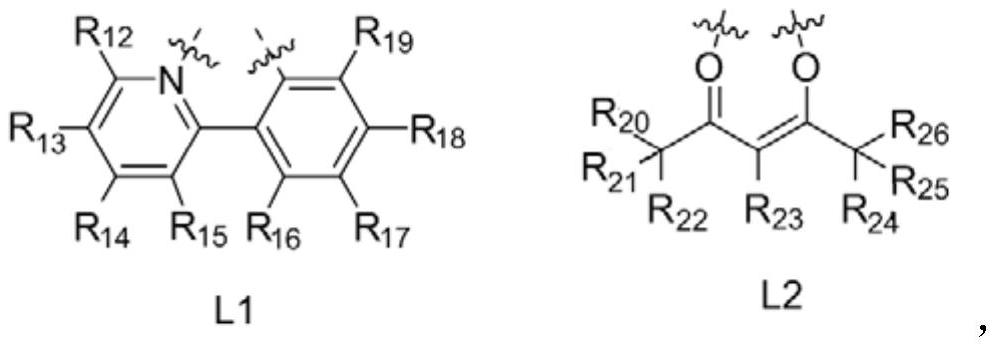
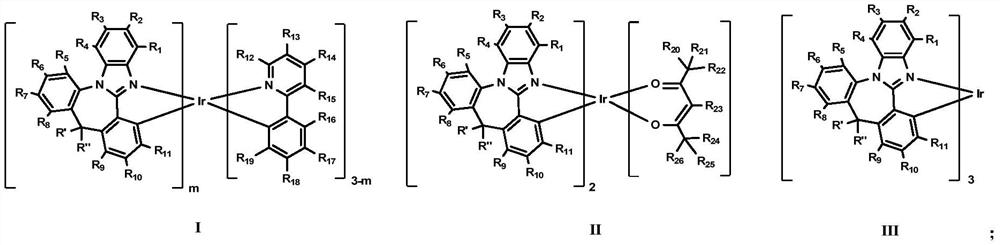
Iridium complex containing benzimidazole structure and application thereof
A technology of benzimidazole and iridium complexes, applied in the field of organic electroluminescence display, can solve the problems of poor stability and life, hindering commercialization, and low phosphorescence efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Synthesis of Ligand P1
[0071]
[0072] The synthetic route is as follows:
[0073]
[0074] The specific experimental steps are:
[0075] (1) In a 2L three-necked flask equipped with mechanical stirring, add 2-fluoronitrobenzene (14.1g, 0.1mol), 2-bromoaniline (25.7g, 0.15mol), stir, and heat up to 180 ℃, heat preservation reaction for more than 30 hours, during the reaction process, the color gradually turns red, and finally turns dark red gradually. After the reaction, the organic phase was separated, extracted, dried, column chromatographed, and the solvent was spin-dried to obtain 24.8 g of orange-red solid M1 with a yield of 85%.
[0076] (2) In a 1L three-neck flask equipped with mechanical stirring, add M1 (29.2g, 0.1mol), sodium sulfide nonahydrate (96g, 0.4mol), ethanol (200mL), water (100mL), nitrogen protection, and heat to Reflux, reflux reaction for 3 hours, end the reaction. The organic phase was separated, extracted, dried, column c...
Embodiment 2
[0081] Example 2: Synthesis of Ligand P2
[0082]
[0083] With reference to the synthesis steps of Example 1, just replace o-bromobenzoyl chloride with 2-bromo-4-methylbenzoyl chloride in step (3), and other raw materials and steps are the same as in Example 1 to obtain ligand P2.
[0084] Product MS (m / e): 324.16; Elemental analysis (C 23 h 20 N 2 ): theoretical value C: 85.15%, H: 6.21%, N: 8.63%; measured value C: 85.31%, H: 6.43%, N: 8.75%.
Embodiment 3
[0085] Example 3: Synthesis of Ligand P3
[0086]
[0087] Referring to the synthesis steps of Example 1, except that 2-bromo-4-methylaniline is used instead of 2-bromoaniline in step (1), other raw materials and steps are the same as in Example 1 to obtain ligand P3.
[0088] Product MS (m / e): 324.42; Elemental analysis (C 23 h 20 N 2 ): theoretical value C: 85.15%, H: 6.21%, N: 8.63%; measured value C: 85.22%, H: 6.36%, N: 8.47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com