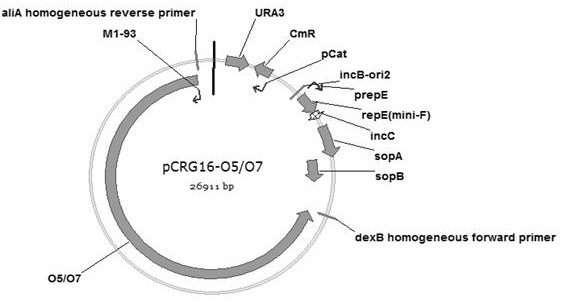
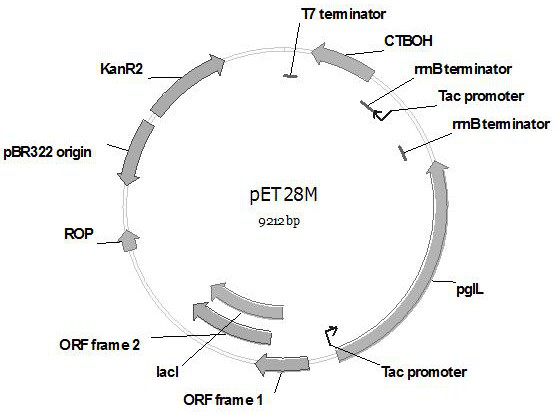
Construction and application of genetically engineered escherichia coli of group of extraintestinal pathogenic escherichia coli glycoprotein conjugate vaccines
A technology of Escherichia coli and conjugated vaccines, applied in the field of synthetic biology, can solve problems such as time-consuming, affecting the stability between batches, and the position of product coupling is not clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] gene acquisition
[0127] In this example, the obtained source from Vibrio cholerae ( Vibbrio cholerae ) codon-optimized cholera toxin B from E. coli (CTB, GI: 877850); and from Neisseria meningitidis ( Campylobacter jejuni ) and an E. coli codon-optimized O-glycosyltransferase encoding gene (undecaprenyl-diphosphooligosaccharide--protein glycotransferase, GI: 905417).
Embodiment 2
[0129] Design of Gene Deletion Primers
[0130] This example uses the CRISPR-cas9 λRed recombination system to delete E.coli The gene of K-12 MG1655 starts with waaL Taking gene as an example, the steps of gene knockout are explained in detail, and the design principles of other gene deletion primers are the same.
[0131] find E.coli K-12 MG1655 waaL nucleotide sequence, designed waaL deletion primers and identification primers. The deletion primers for waaL are GwaaL-F, HwaaL-GwaaL-R, waaL-up-F, waaL-up-Rm, waaL-down-Fm, waaL-down-R, HwaaL-pTarget-backbone-F, GwaaL-pTarget -backbone-R identified primer as S2-waaL-F / R. Nucleotide sequences are shown in Tables 1-8.
Embodiment 3
[0133] 3.1 E.coli K-12 MG1655 Δ waaL build
[0134] (1) Extract pCas plasmid
[0135] The pCas plasmid was extracted with a Sanitary Column Plasmid Mini-Extraction Kit, and the specific steps were shown in the kit instructions.
[0136] (2) Preparation E.coli K-12 MG1655 competent cells
[0137] (3) E.coli Acquisition of K-12 MG1655 / pCas Strain
[0138] The extracted pCas plasmid was transformed into E.coli K-12 MG1655 competent cells were then transferred to recovery medium for about half an hour, coated with kanamycin-resistant 2YT plates, and cultured overnight in a 30°C incubator. The next day, it can be seen that the single colony growing on the plate is the E.coli K-12 MG1655 strain containing the pCas plasmid.
[0139] 3.2 Construction of pTarget-waaL plasmid
[0140] (1) Principles of gRNA design: Open http: / / www.rgenome.net / cas-designer / PAM Type to select SpCas9 from Streptococcus pyogenes: 5'-NGG-3' (because Cas9 protein comes from Streptococcus py...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap