Terpene glycoside derivatives and uses thereof
A technology for terpene glycosides and terpene glycosides, applied in the field of terpene glycosides, can solve problems such as bad taste attributes, preventing widespread adoption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Generation of mono-α-1,6-glucosylated stevioside compounds (compounds I and II)
[0118] At room temperature, rubusoside (2 g) and corn maltodextrin (2 g) with a dextrose equivalent (DE) of 18 were dissolved in 10 ml of NaOAc-HOAc (pH=6.0, 0.2 M, 10 mL) buffer or removed ionized water. Subsequently, 100 µl of transglucosidase L (Amano) was added to the mixture. The enzyme-containing mixture was then heated to 60°C and incubated at 60°C for 24 hours to allow the transglucosidation reaction to proceed, resulting in a glucosidase with a single α-1,6 glucosidic bond. Glycosylated terpene glycosides. The reaction was stopped by inactivating the transglucosidase by incubating the reaction mixture at 100°C for 30 minutes.
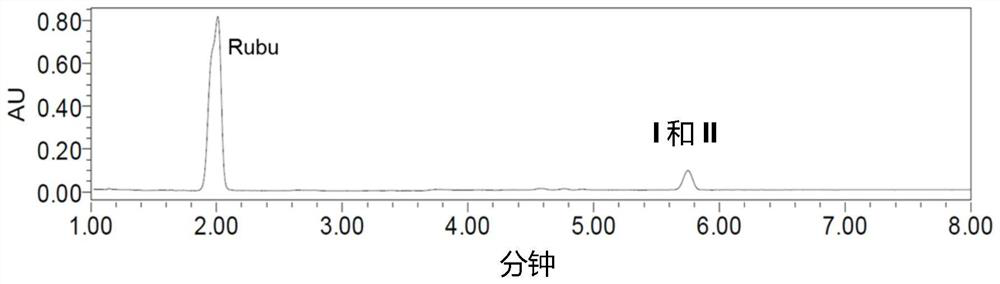
[0119] The resulting reaction mixture was analyzed by UPLC-UV and a mixture containing glucosylated terpene glycosides with a single α-1,6 glucosidic linkage was identified (see figure 1 ). The identified mixture was purified by preparative...
Embodiment 2
[0120] Example 2: Generation of mono-alpha-1,6-glucosylated stevioside compounds (compounds I and II) by methods according to some morphologies presented herein, using rubusoside and maltose as starting materials
[0121] Rubusoside (2 g) and maltose (2 g) were dissolved in 10 ml of NaOAc-HOAc (pH=6.0, 0.2 M, 10 mL) buffer or deionized water at room temperature. Subsequently, 100 μL of transglucosidase L (Amano) was added to the mixture. The enzyme-containing mixture was then heated to 60°C and incubated at 60°C for 24 hours to allow the transglucosidation reaction to proceed, resulting in a glucosidase with a single α-1,6 glucosidic bond. Glycosylated terpene glycosides. The reaction was stopped by inactivating the transglucosidase by incubating the reaction mixture at 100°C for 30 minutes.
[0122] The resulting reaction mixture was analyzed by UPLC-UV and a mixture containing glucosylated terpene glycosides with a single α-1,6 glucosidic linkage was identified (see figu...
Embodiment 3
[0123] Example 3: Generation of mono-alpha-1,6-glucosylated rebaudioside A (Compound III) by methods according to some morphologies presented herein, using rebaudioside as starting material
[0124] Rebaudioside A (2 g) and corn maltodextrin (2 g) with a dextrose equivalent (DE) of 18 were dissolved in 10 ml of deionized water at room temperature. Subsequently, 100 µl of transglucosidase L (Amano) was added to the mixture. The enzyme-containing mixture was then heated to 60°C and incubated at 60°C for 24 hours to allow the transglucosidation reaction to proceed, resulting in a glucosidase with a single α-1,6 glucosidic bond. Glycosylated terpene glycosides. The reaction was stopped by inactivating the transglucosidase by incubating the reaction mixture at 100°C for 30 minutes.
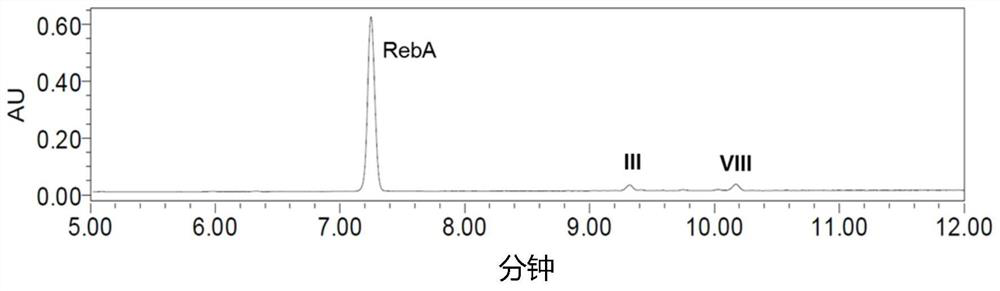
[0125] The resulting reaction mixture was analyzed by UPLC-UV, and glucosylated terpene glycosides with a single α-1,6 glucosidic linkage were identified (see image 3 ). The identified mixture was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com