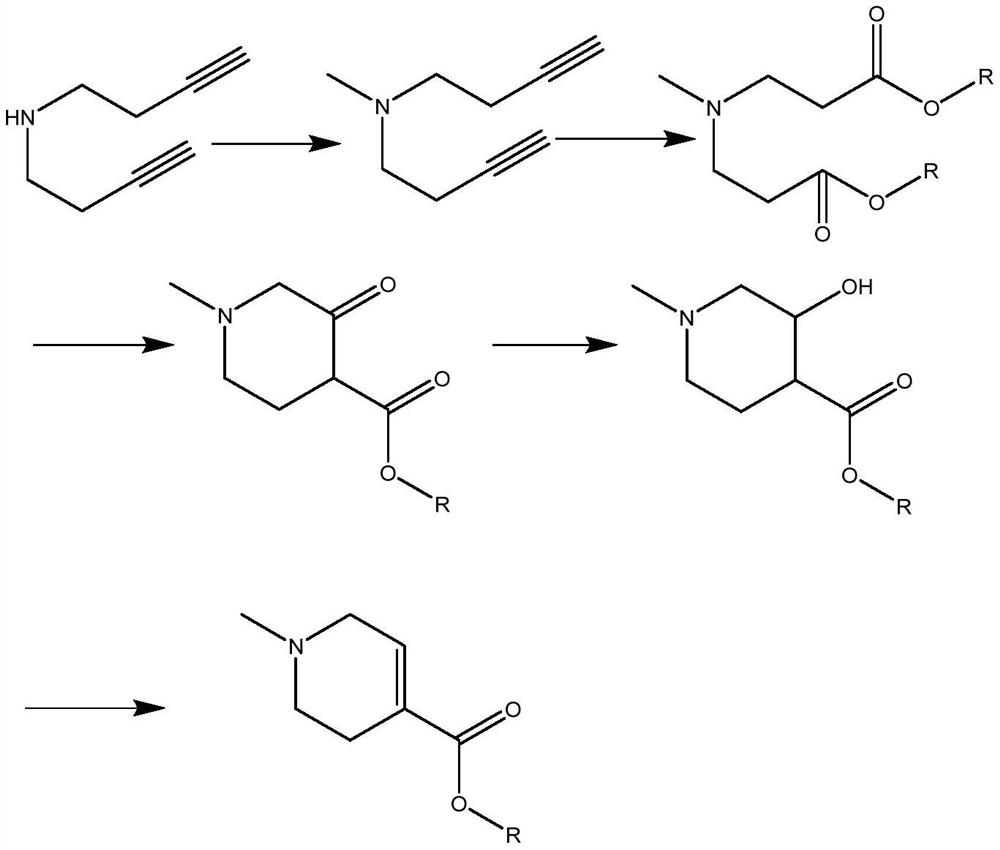
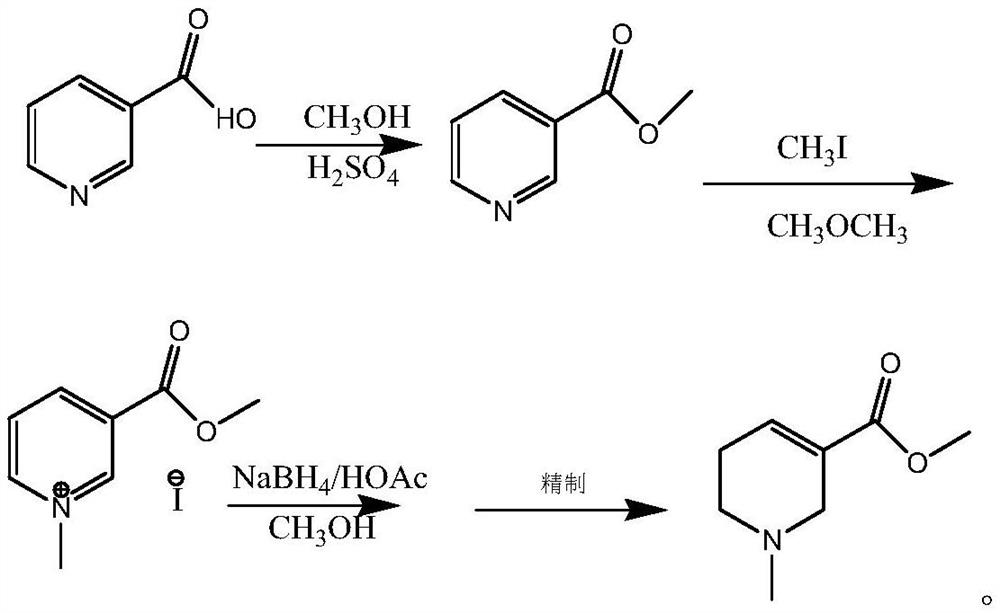
A kind of synthetic method of arecoline
A synthesis method and technology of arecoline are applied in the field of synthesis of arecoline, can solve the problems of high toxicity of methyl iodide, low single-step yield and high price, and achieve the effects of simple preparation method, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
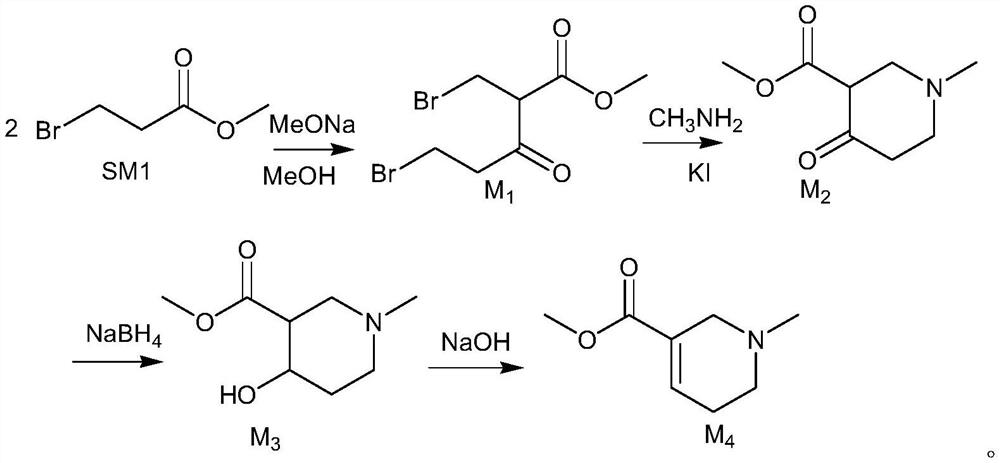
[0033] 1) Synthesis of M1
[0034]
[0035] Add 50g (0.299mol) methyl 3-bromopropionate, 54g methanol solution of sodium methoxide (30wt%) and 150ml methanol to the reaction flask, heat under reflux for 5 hours, cool down to 20-30°C after completion, add 15g formic acid dropwise , 1.5 hours of dropwise addition, stirring for half an hour, suction filtration (precipitated impurities, such as sodium formate), washing the filter cake to obtain the methanol solution of M1.
[0036] 2) Synthesis of M2
[0037]
[0038] M1 methanol solution, 28 g methylamine methanol solution (30 wt %) and 0.4 g KI were added to the reaction flask, and the reaction was carried out at 35-45° C. for 7.5 h. After the reaction was completed, the temperature was lowered to 10-20°C, 15ml of water was added, extracted with dichloromethane, washed with 15ml of 10% dilute hydrochloric acid, washed with 15ml of saturated brine, distilled under reduced pressure with dichloromethane to dryness to obtain ...
Embodiment 2
[0046] 1) Synthesis of M1
[0047] Add 50g (0.299mol) methyl 3-bromopropionate, 74g sodium methoxide methanol solution (30wt%), 150ml methanol into the reaction flask, heat under reflux for 6 hours, cool down to 20-30°C after completion, add 15g formic acid dropwise, The dropwise addition was completed in 1 hour, and after stirring for half an hour, suction filtration was performed, and the filter cake was washed to obtain a methanol solution of M1.
[0048] 2) Synthesis of M2
[0049] M1 methanol solution, 28 g methylamine methanol solution (30 wt %), 0.4 g KI were added to the reaction flask, and the reaction was carried out at 35-45° C. for 7 h. After the reaction was completed, the temperature was lowered to 10-20° C., 15 ml of quantitative water was added, extracted with dichloromethane, washed with 15 ml of 10% dilute hydrochloric acid solution, washed with saturated brine, and distilled to dryness with dichloromethane to obtain 38.7 g of compound M2, yield: 75.59%; Pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com