Anti-PD-L1/CD47 bispecific antibody and use thereof
A bispecific antibody, PD-L1 technology, used in the field of biomedicine or biopharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Example 1: Molecular structure design and expression of PD-L1 / CD47 bispecific antibody
[0200] The inventors designed bispecific antibodies that simultaneously bind human PD-L1 and CD47 extracellular domains. Among them, the PD-L1 single domain antibody sequence is derived from Chinese patent CN201810151835.5, and the CD47 single domain antibody sequence is derived from Chinese patent CN201810151752.6.
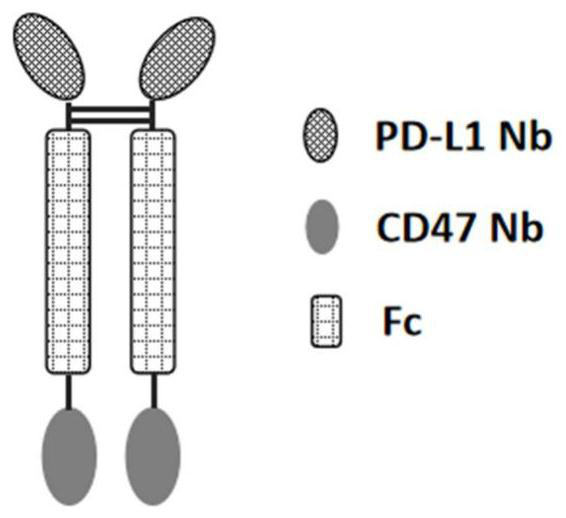
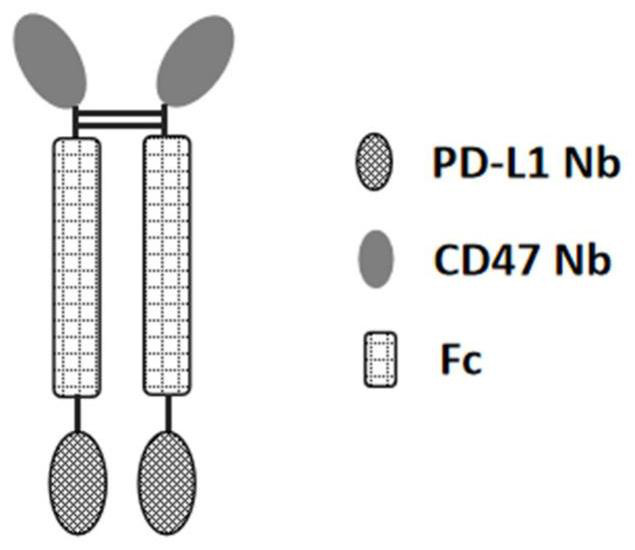
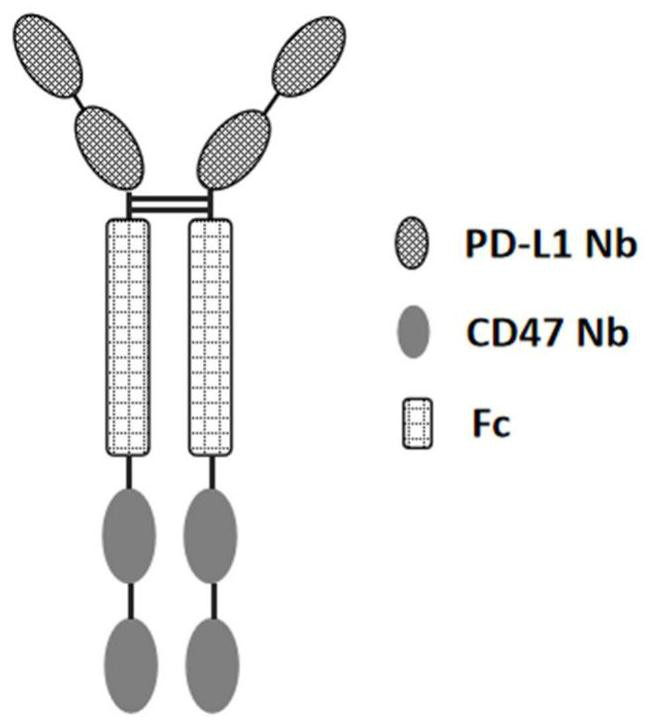
[0201] The present invention designs a variety of PD-L1 / CD47 bispecific antibody molecules with different structures, and measures their activity. This example only exemplifies the bispecific antibody molecules with four structures, which are as follows: Figure 1A The indicated double antibody A, such as Figure 1B The double antibody B shown, such as Figure 1C Double Antibody C as indicated and as Figure 1D In the shown double antibody D, double antibody A and double antibody B are in the form of tetravalent antibodies, and double antibody C and double antibody D...
Embodiment 2
[0205] Example 2: Comparison of blocking activity of PD-L1 / CD47 bispecific antibody on CD47 / SIRPa pathway
[0206] (1) Each sample takes 5×10 5 In 0.5% BSA-PBS buffer, several Jurkat cells (highly expressing human CD47) were added with serially diluted PD-L1 / CD47 bispecific antibody and positive control CD47 single domain antibody Fc fusion protein (Nb-Fc), antibody dilution gradient For 514nM, 257nM, 129nM, 64.3nM, 32.1nM, 16.1nM, 8.03nM, 4.02nM, 2.00nM, 1.00nM, 0.50nM, 0.25nM, add 100uL to each sample, and add 5ug hSIRPa(ECD) to all samples at the same time -Fc-Biotin, incubated at 4°C for 20 min; (2) Wash the cells twice with PBS, add eBioscience SA-PE, incubate at 4°C for 20 min, wash the cells twice with PBS, and detect them with a flow cytometer (BD FACS Calibur). Graphpad Prism 6 software was used for data processing.
[0207] The result is as figure 2 Shown: The blocking activity of the octavalent antibody is better than that of the tetravalent antibody, and the te...
Embodiment 3
[0208] Example 3: Comparison of blocking activity of PD-L1 / CD47 bispecific antibody on PD-1 / PD-L1 pathway
[0209] Take 3×10 for each sample 5 A375 / PD-L1 stably transfected cells were added to 0.5% BSA-PBS buffer, and the double antibody to be tested and the control antibody PD-L1 single domain antibody Fc fusion protein (Nb-Fc) were added in a serial dilution, and the antibody dilution gradient was 184nM, 91.8nM, 45.9nM, 22.9nM, 11.5nM, 5.74nM, 2.87nM, 1.43nM, 0.72nM, 0.36nM, 0.18nM, 0.09nM, 100uL was added to each sample, and 3ug hPD-1(ECD )-Fc-Biotin, incubated at 4°C for 20 min; (2) Wash the cells twice with PBS, add the SA-PE of eBioscience, incubate at 4°C for 20 min, wash the cells twice with PBS, and detect them with a flow cytometer (BDFACS Calibur). Graphpad Prism 6 software was used for data processing.
[0210] The result is as image 3 As shown: the bispecific antibodies with different structures have lower PD1 / PD-L1 interaction blocking activity than the origi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com