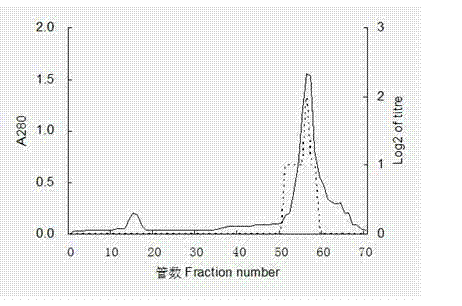
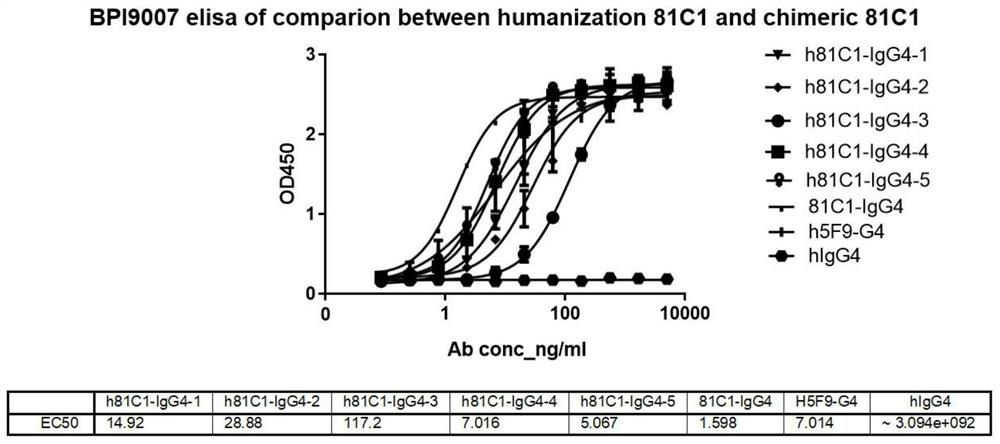
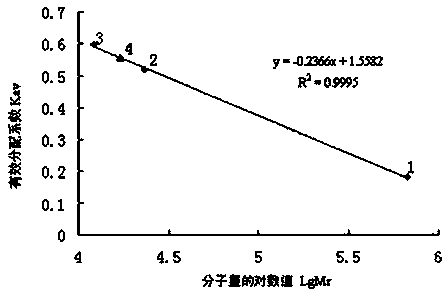
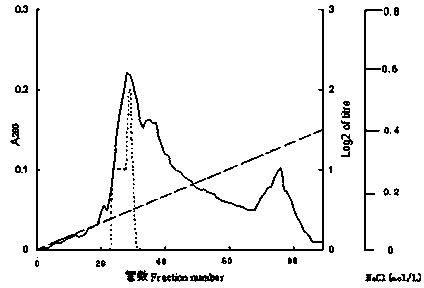
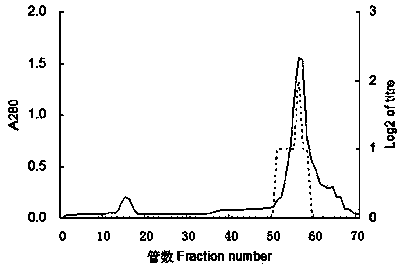
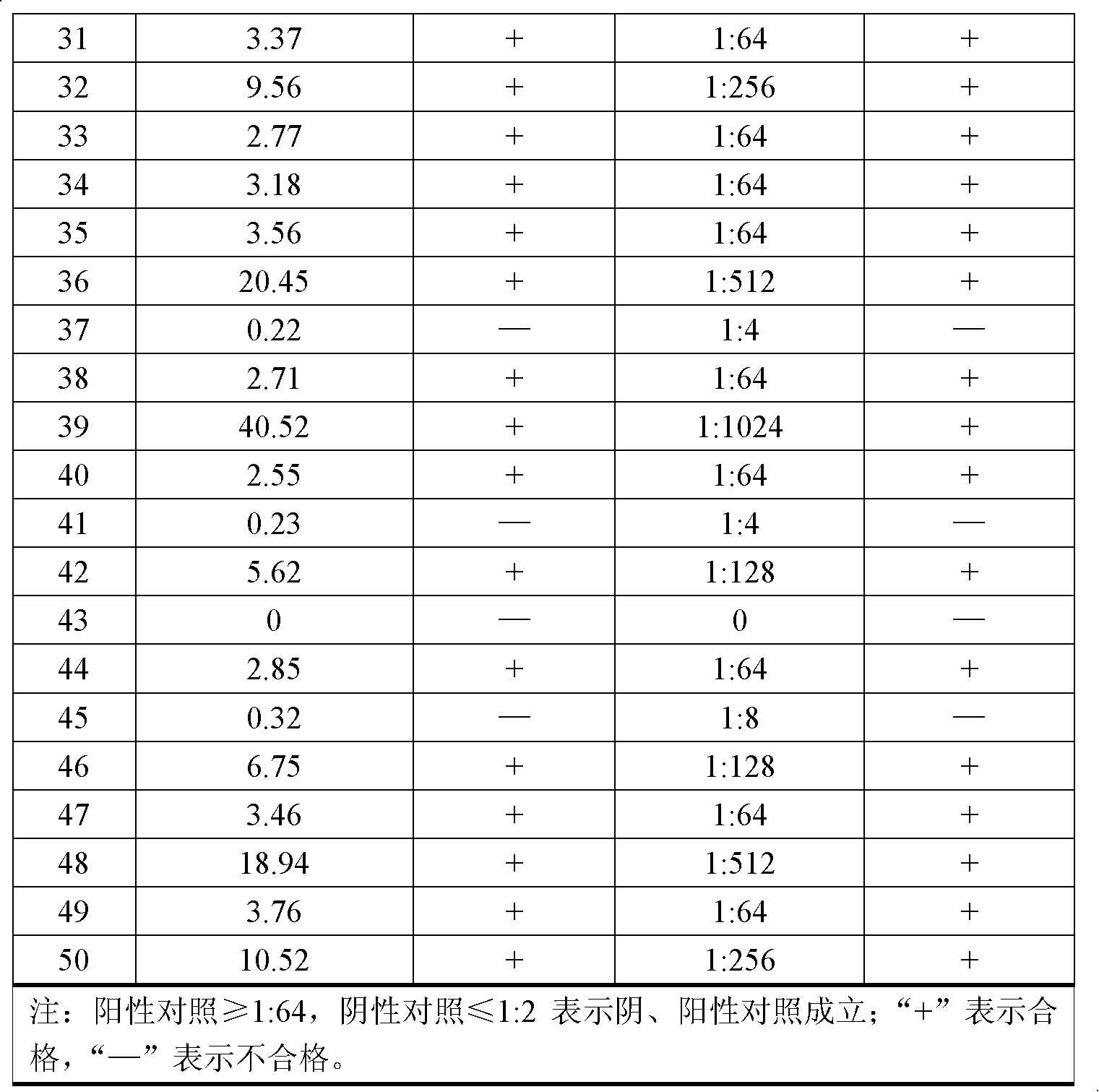
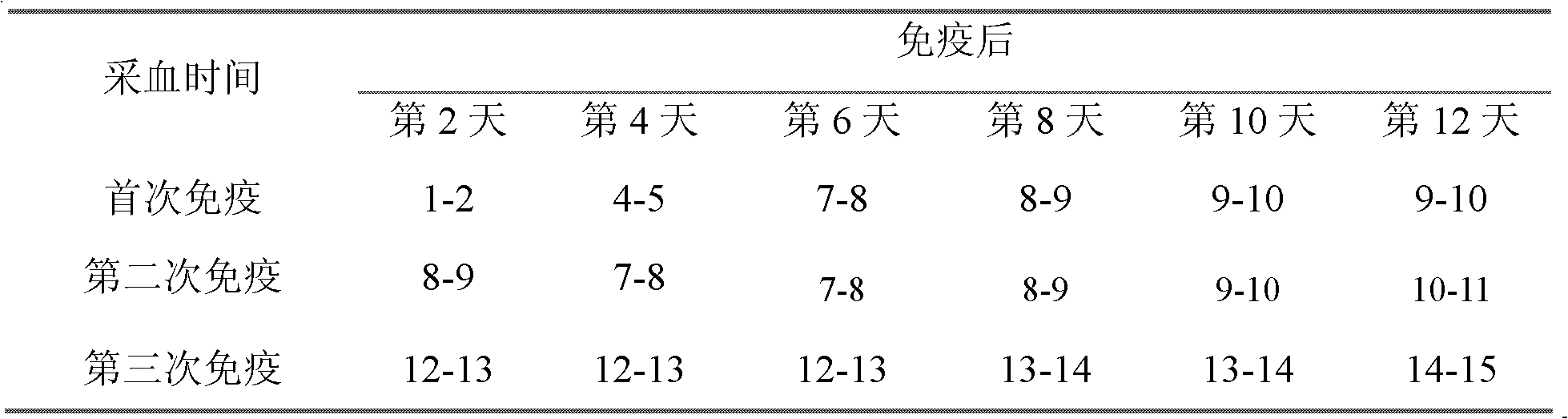
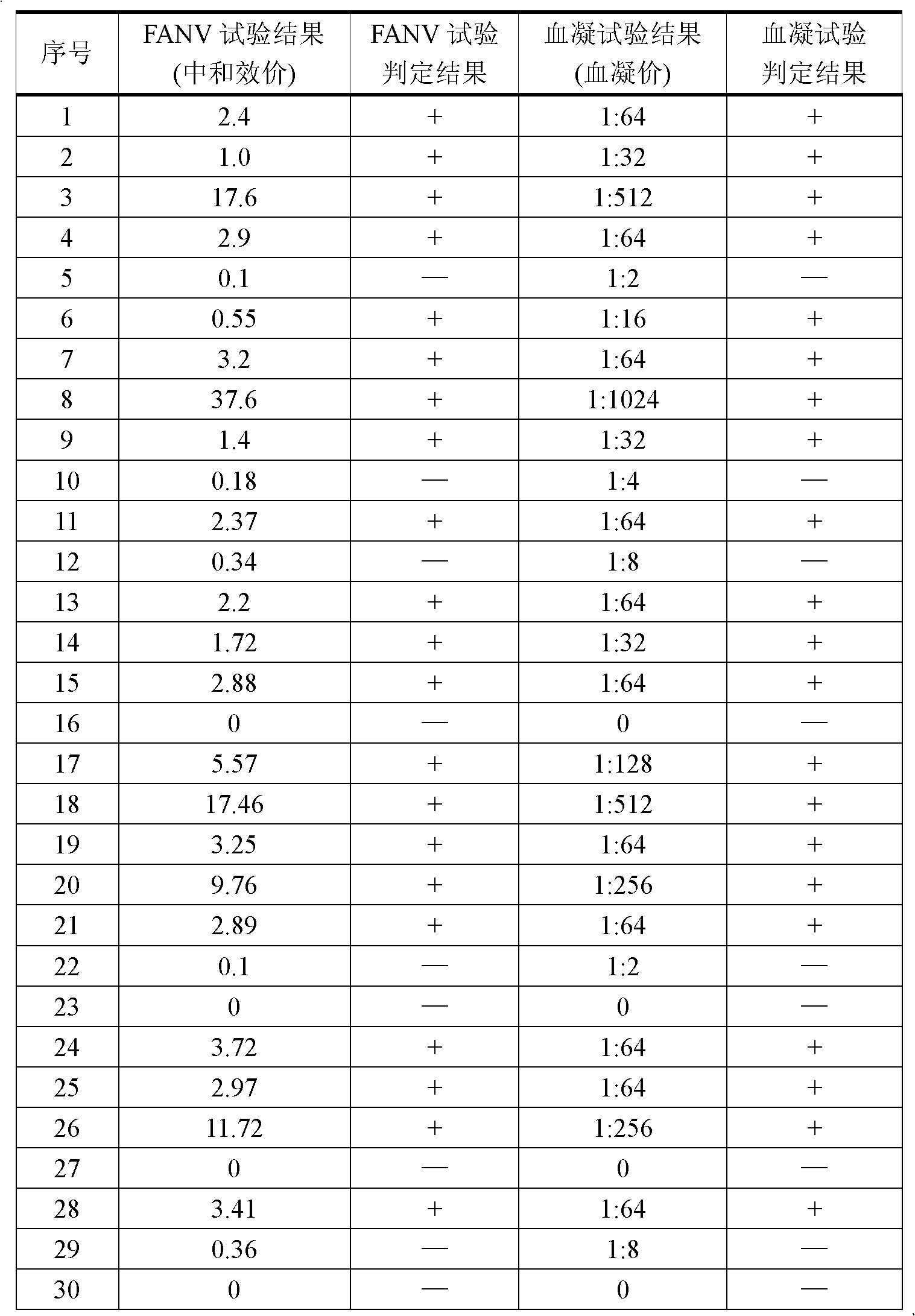Patents
Literature
37 results about "Red blood cell agglutination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
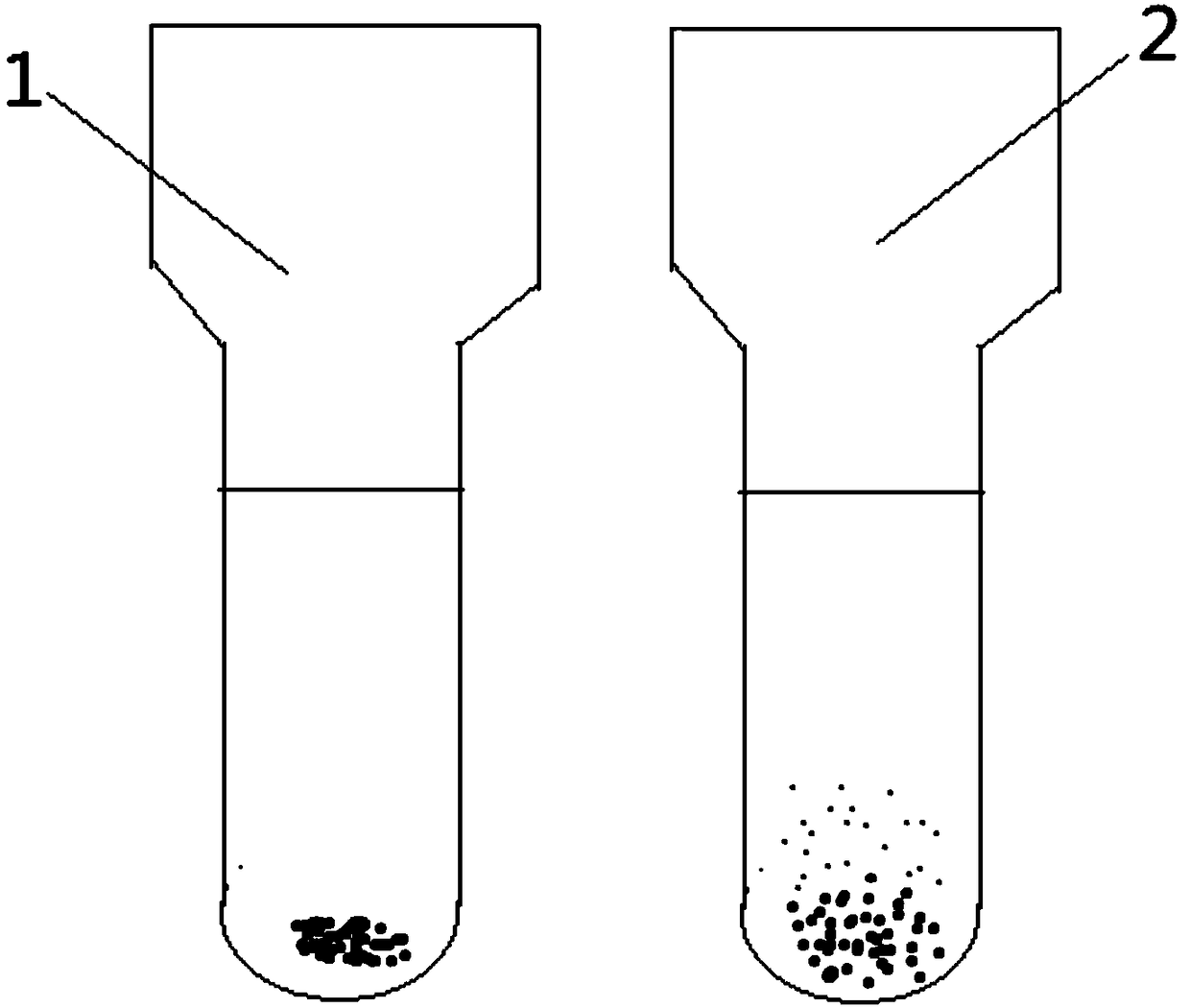
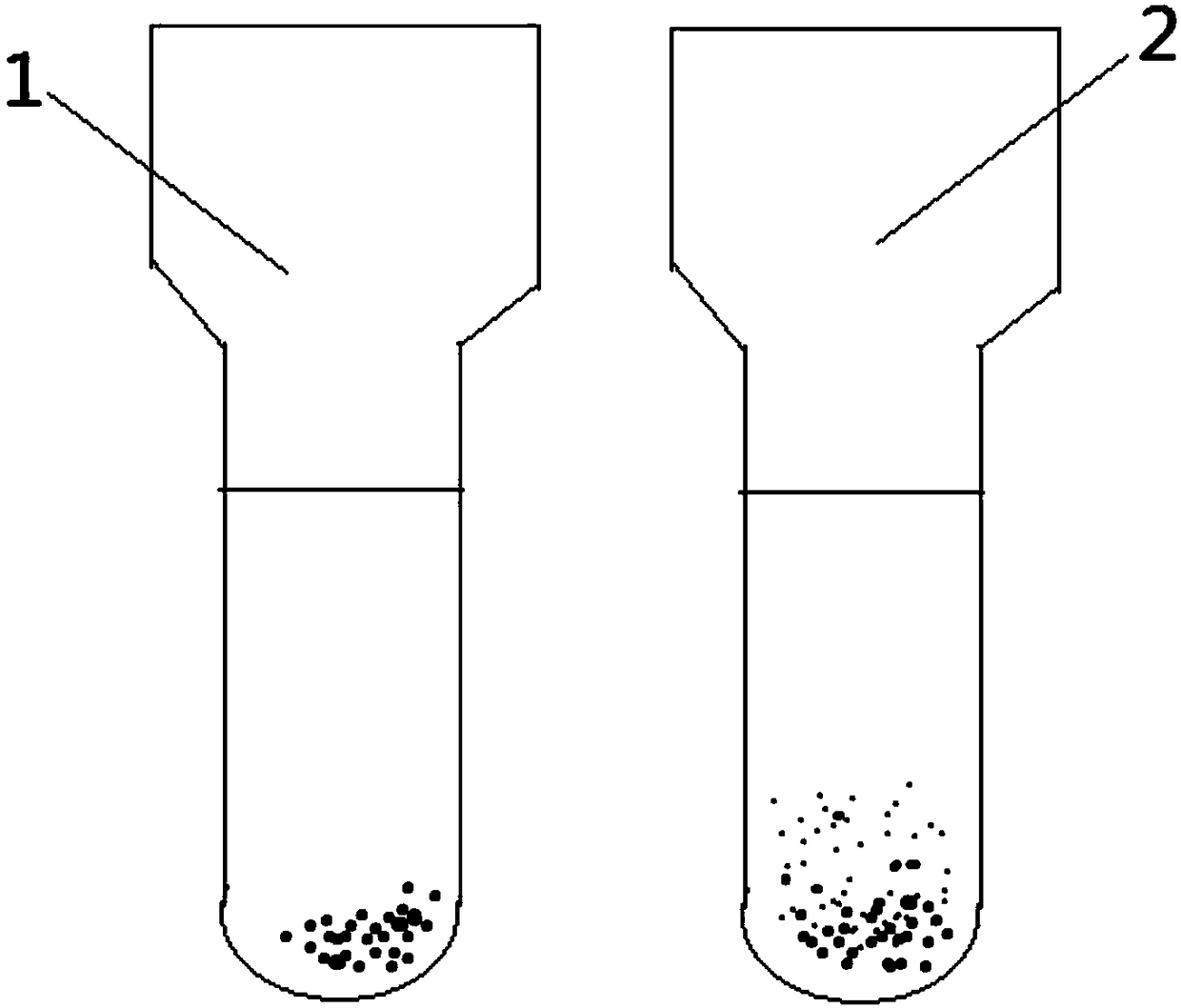
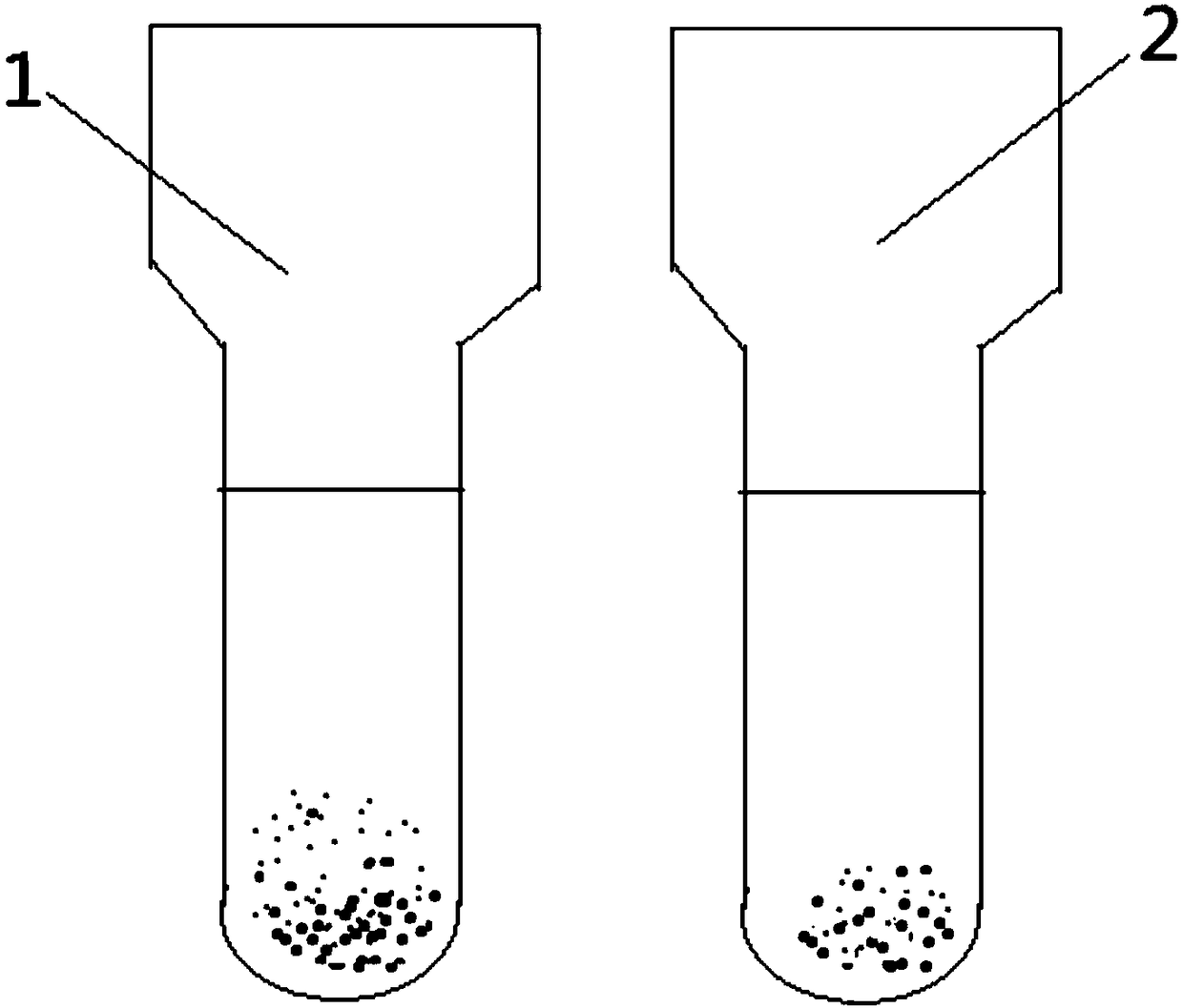
•Clumping (agglutination) of red blood cells is frequently caused by cold agglutinins. Cold agglutinins are IgM antibodies that may arise following viral or Mycoplasma infections, or in the setting of plasma cell or lymphoid neoplasms. Agglutination of red cells can interfere with red blood cell indices.
Biosensor and preparation method and application thereof
InactiveCN104931560AEffective filteringReduce sensitivityMaterial electrochemical variablesRed blood cellRed blood cell agglutination
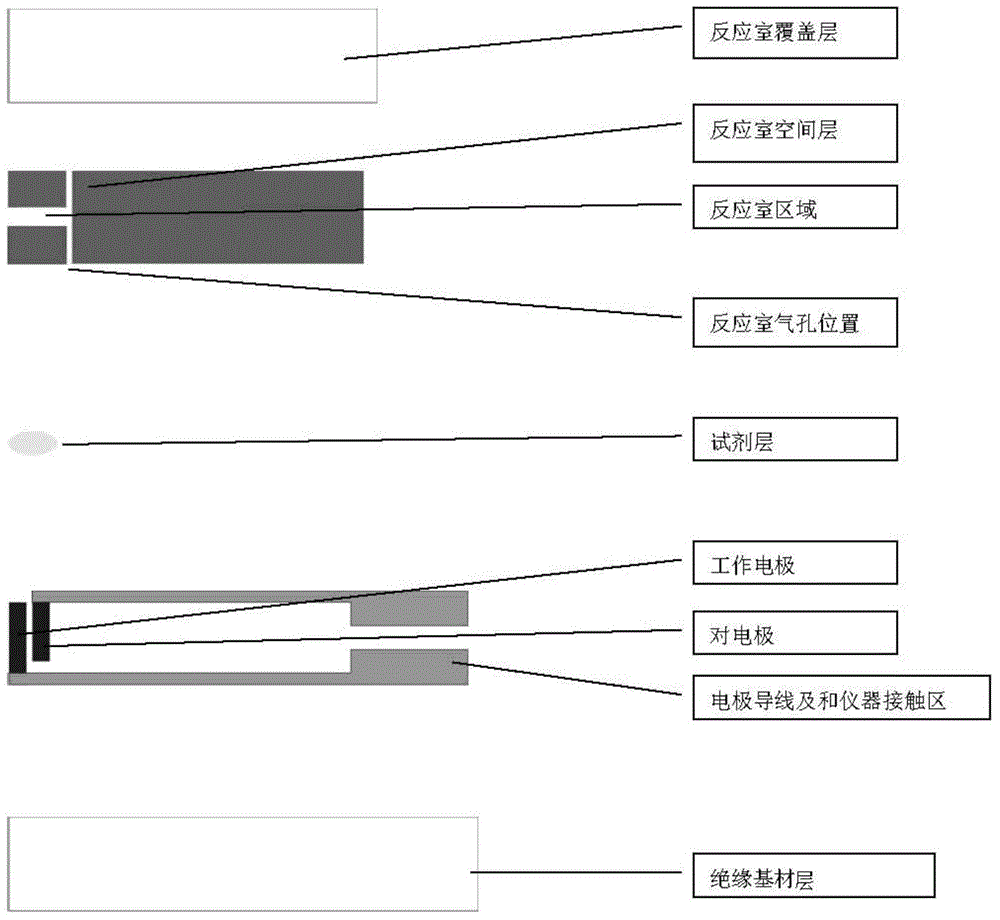
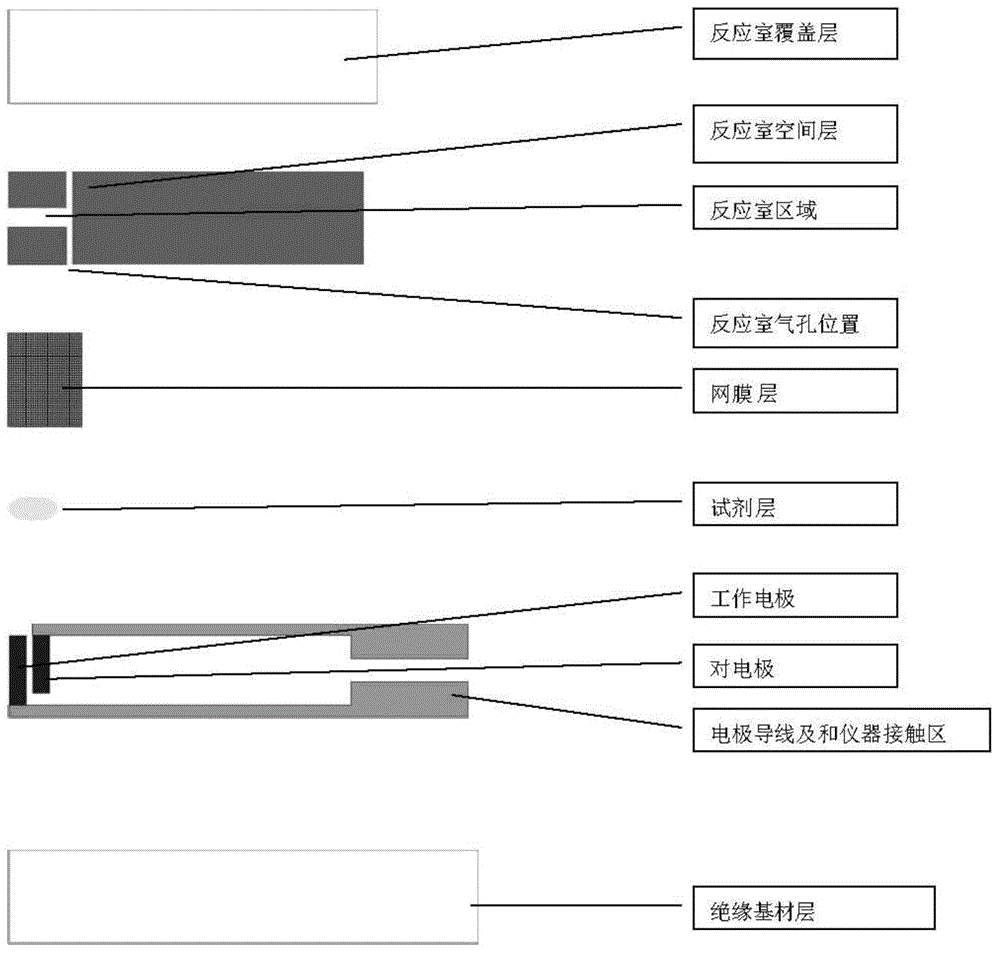
The invention relates to the field of biological detection instruments, in particular to a biosensor. The biosensor comprises a reagent layer. The reagent layer contains red blood cell agglutinant or an omentum layer adhering to the reagent layer. The omentum layer is treated through the red blood cell agglutinant. The invention further provides a preparation method and application of the biosensor. As the problem of the sensibility of the hematocrit value is not solved completely in the prior art, the problem of the sensibility of the hematocrit value is solved by reducing the sensibility of the hematocrit value; accordingly, the accuracy of testing results is improved, and interference of red blood cells on the testing results is reduced to the maximum extent.
Owner:王天星
Preparation method and application of LcGa recombinant protein of galaptins of larimichthys crocea
InactiveCN105063076AMaintain natural biological activityEasy to prepareAntibacterial agentsPeptide/protein ingredientsBiotechnologyEscherichia coli
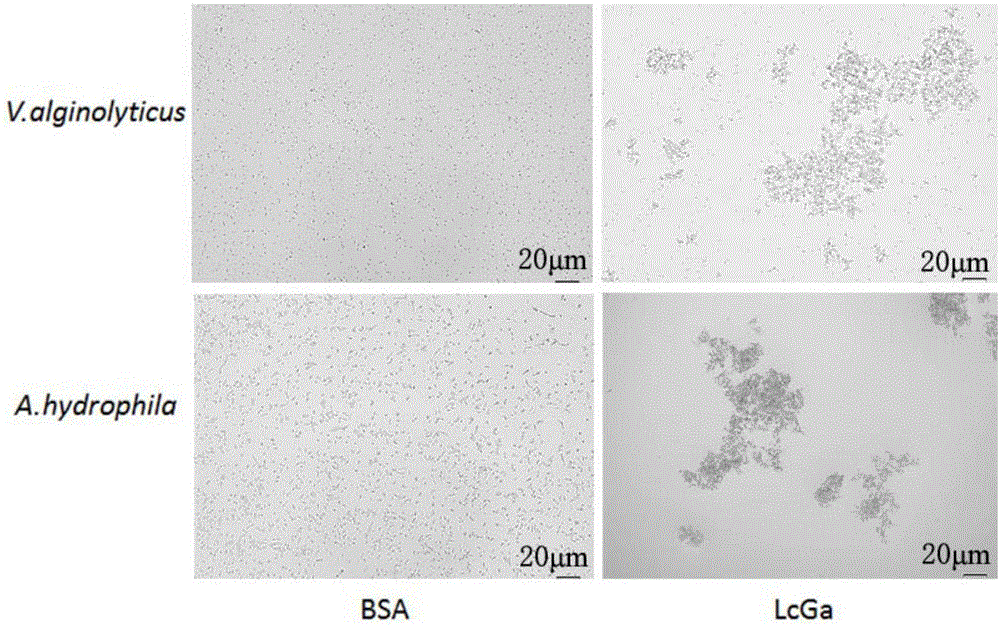
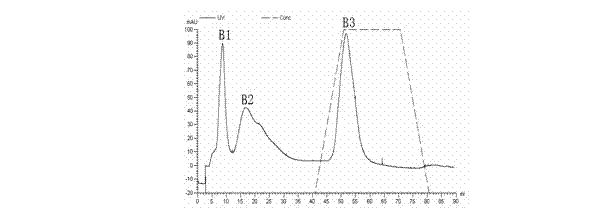
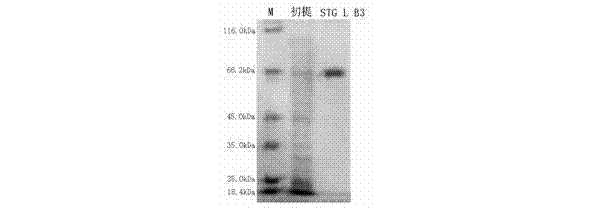
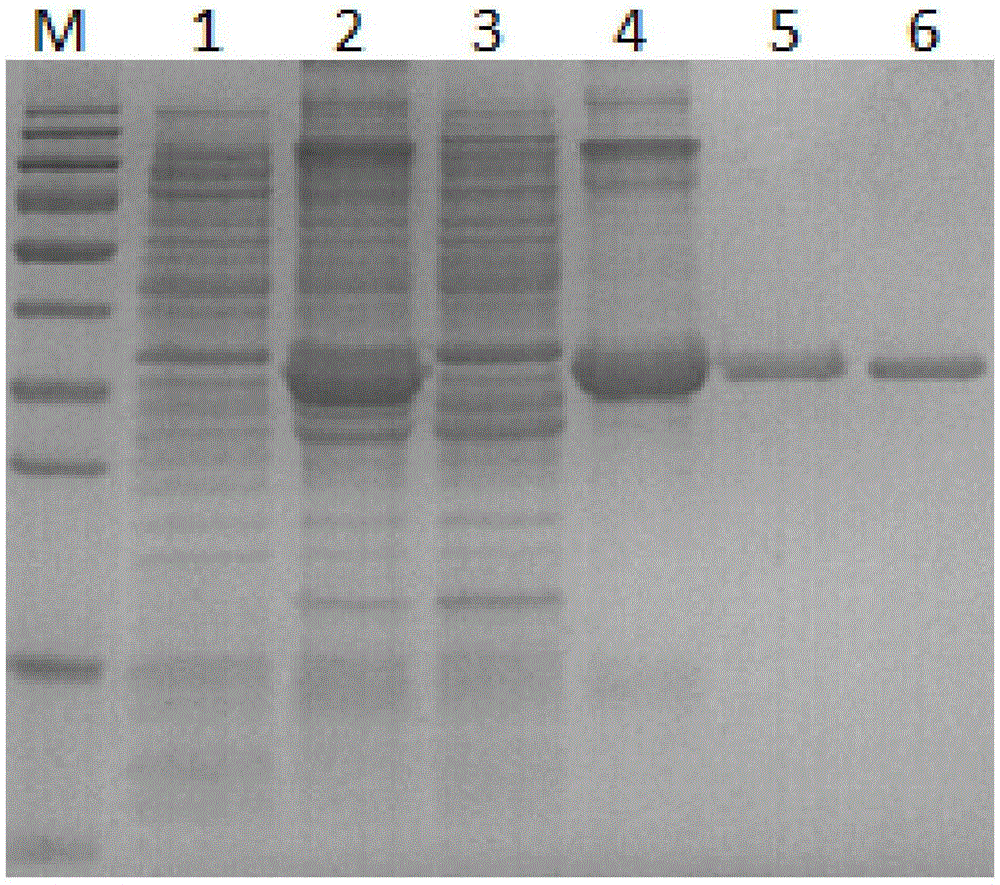
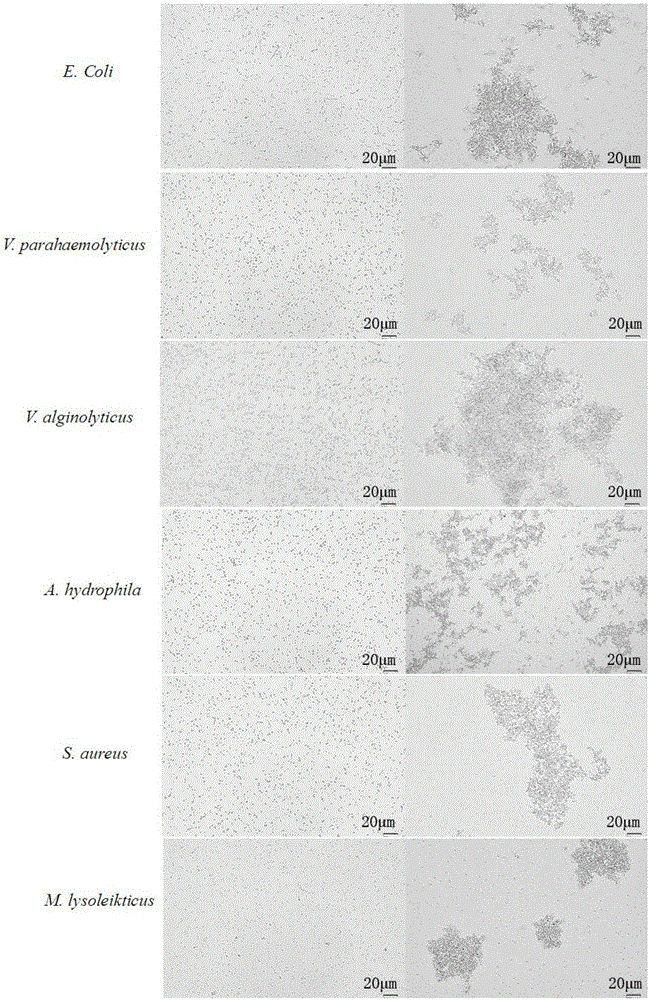
The invention provides a preparation method and an application of LcGa recombinant protein of galaptins of larimichthys crocea, and relates to genetic engineering. The preparation method comprises steps as follows: 1), a pGEX-6P1-LcGa recombinant expression vector is constructed; 2), an escherichia coli expression strain pGEX-6P1-LcGa-BL21 with high-copy transformants is acquired; 3), fermented cultivation and induced expression of the transformants are performed; the recombinant protein LcGa is purified. The LcGa recombinant protein of galaptins of the larimichthys crocea can be applied to mediated bacteria agglutination and mediated red blood cell agglutination, and can be applied to preparation of fish immune additives and bacterial control drugs. The prokaryotic expression recombinant protein exists in a soluble mode, and the natural bioactivity of the recombinant protein is kept; further, the preparation method is simple and easy to operate, the yield is high, the low-cost scale production becomes possible, and a new way is provided for development of the novel fish immune additives and the bacterial control drugs.
Owner:JIMEI UNIV
Malania oleifera lectin and method for preparing same
InactiveCN102174095AAchieve efficient utilizationSimple extraction and purification processPeptide/protein ingredientsAntiviralsUltrafiltrationElectrophoresis
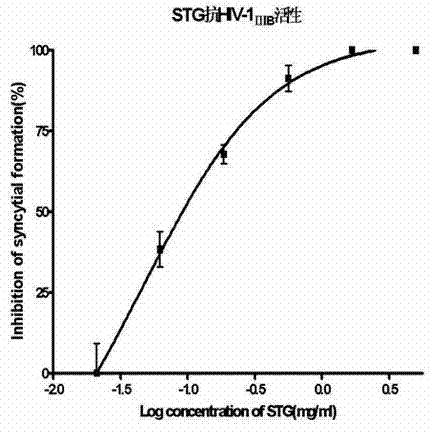
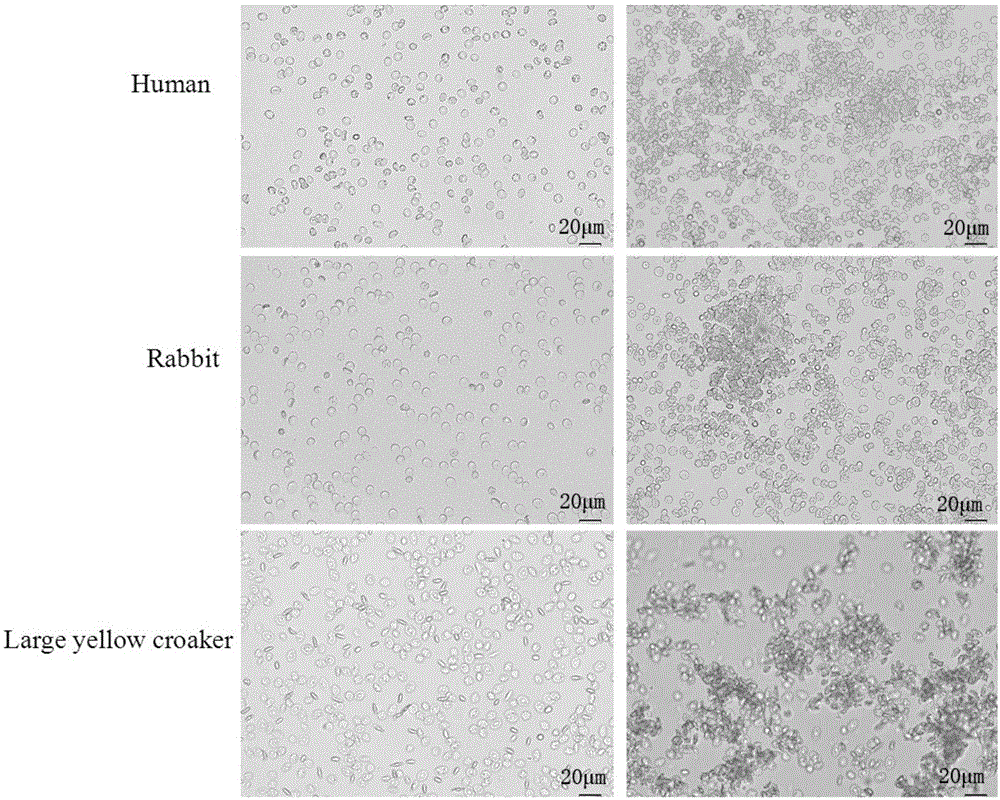
The invention provides malania oleifera lectin and a method for preparing the same. The method comprises the following steps: grinding malania oleifera, stirring and socking the ground malania oleifera into cold water, grading and precipitating by using ammonium sulfate, ultrafiltering to remove salt, concentrating, carrying out ion exchange chromatography, purifying, ultrafiltering to remove salt, concentrating, freezing and drying to obtain the refined malania oleifera lectin. The prepared malania oleifera lectin has the molecular weight of about 60kd, the electrophoresis purity of above 90% and the potency of 1:10<5> or more, namely the HIV infected cell (inhibition concentration 50) IC50 of 1:10<5>, can agglutinate the red blood cells of a rabbit, a pig, a chicken, a duck and a mouse and can be used for preventing HIV infection.
Owner:KUNMING UNIV OF SCI & TECH
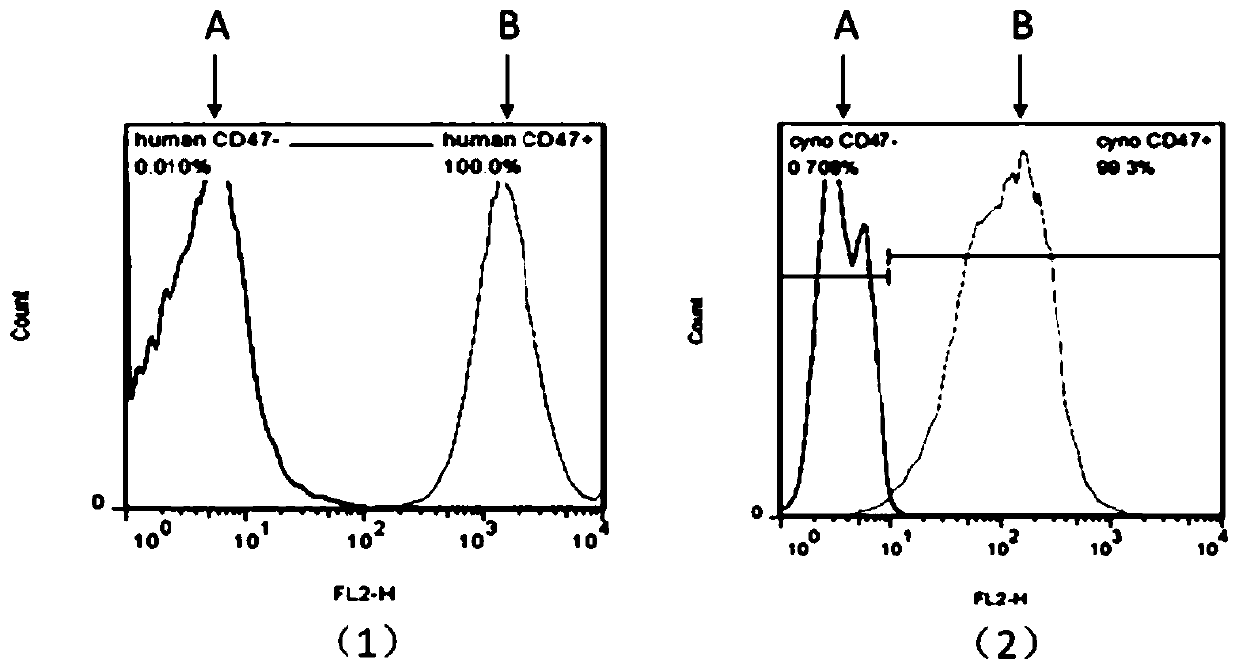
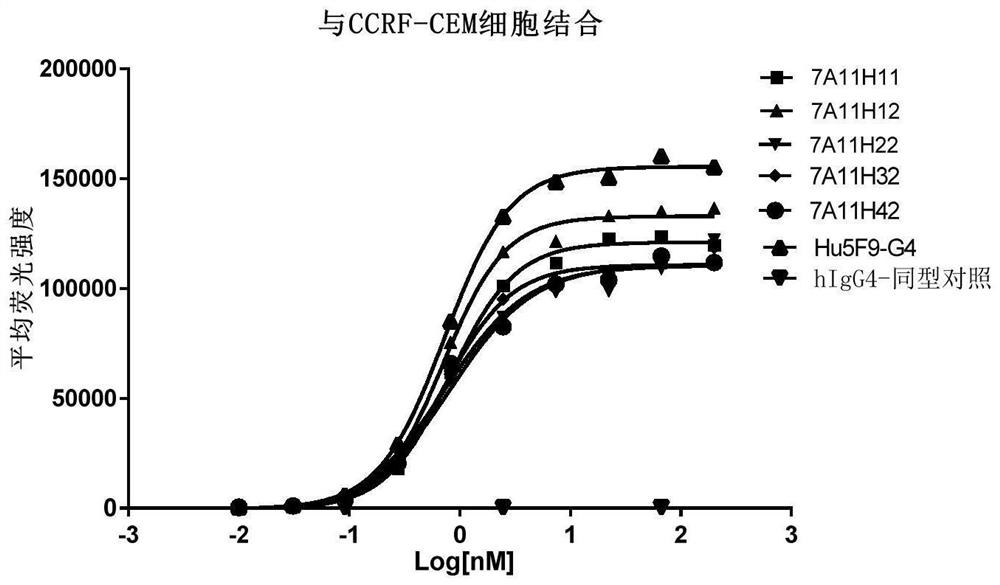
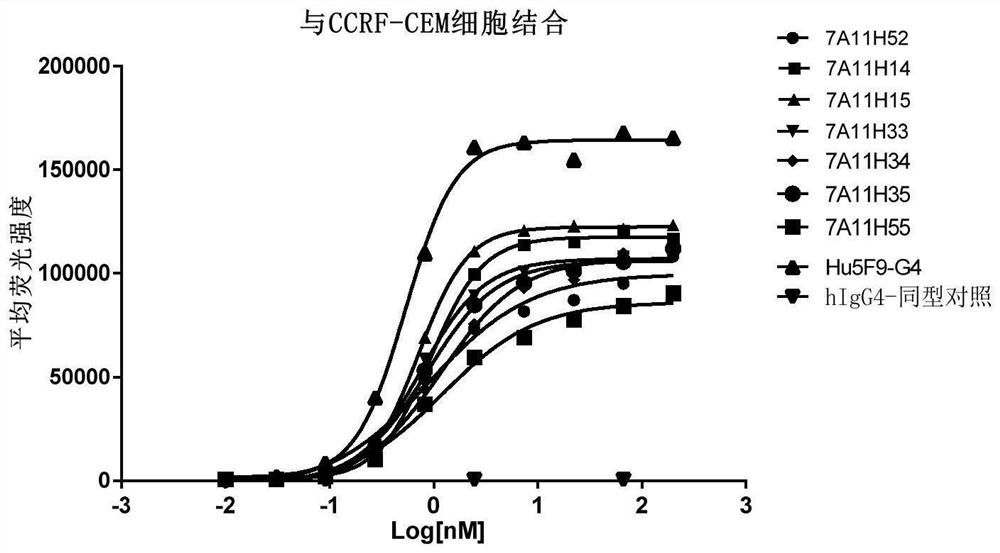
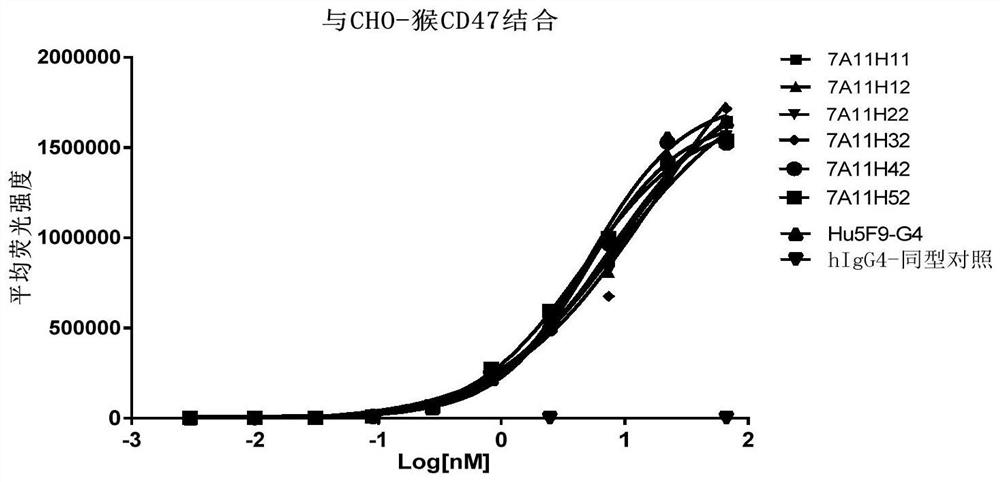
Anti-CD47 antibody and application thereof
ActiveCN110872350AImprove anti-tumor effectLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesAntigen Binding Fragment
The invention relates to the technical field of antibody drugs, in particular to an anti-CD47 antibody or an antigen binding fragment thereof, a pharmaceutical composition containing the anti-CD47 antibody or the antigen binding fragment thereof and their application. The anti-CD47 antibody or the antigen binding fragment thereof provided by the invention has significant antitumor activity, has high affinity with the human CD47 protein, can block the ability of SIRPa binding to CD47 on the cell surface, does not have significant erythrocyte agglutination activity, and can be applied to preparation of antitumor drugs.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Method of detecting red cell antigen-antibody reactions
InactiveUS20080261248A1High test sensitivityEasy to disperseBioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeCellular antigens
A process for the detection of antibodies in a test sample by carrying out the following steps:(a) preparing a suspension of erythrocytes with a test serum or plasma by mixing a test serum or plasma with erythrocytes;(b)incubating the suspension formed in step (a) at a temperature of from 37° C. to 45° C. for a period of time from sixty seconds to 10 minutes to bind any antibodies in the test serum or plasma to the surface of said erythrocytes(c)combining said suspension with an amount of a solution of a macromolecule which is effective for agglutination of said erythrocytes;(d) packing the resultant red cell agglutinates by centrifugation from the suspension of step (c); and,(f) determining the presence of anti-erythrocyte antibodies by observing if antibody-dependent erythrocyte aggregation has occurred.
Owner:CLAVINA DIAGNOSTICS
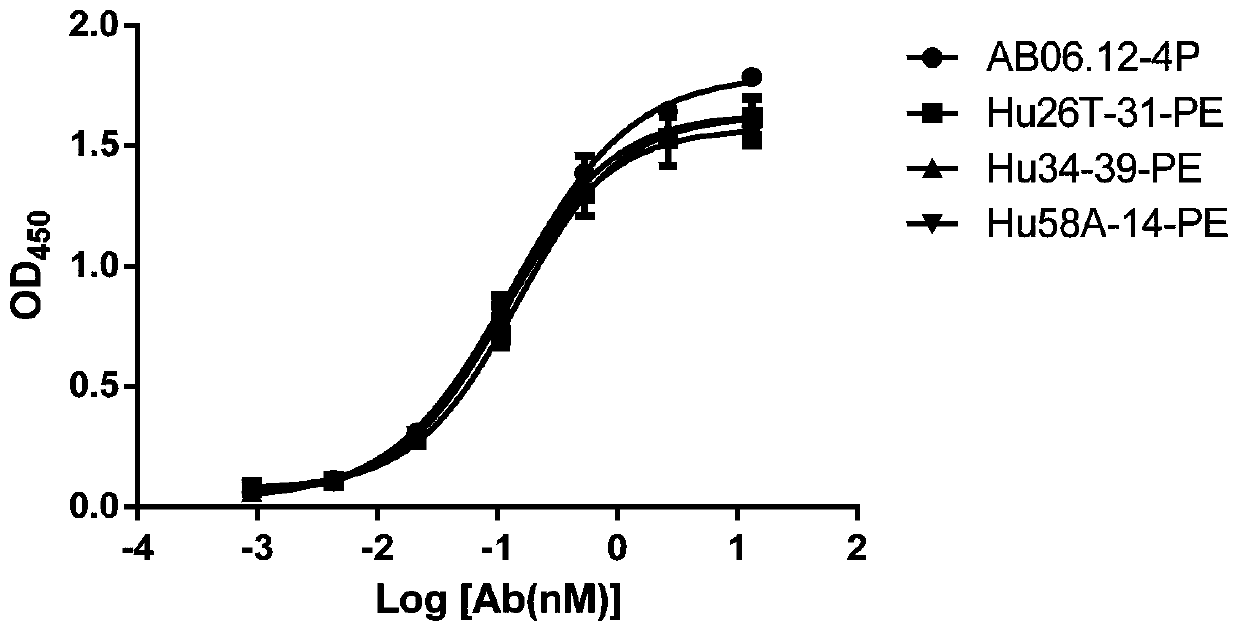
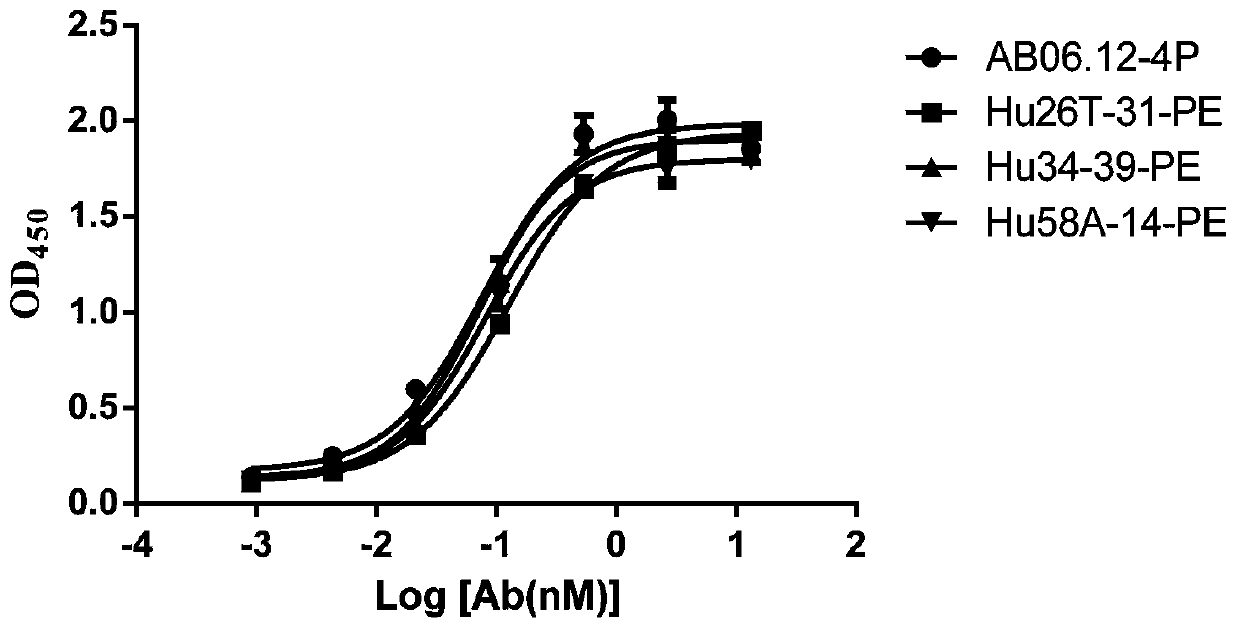
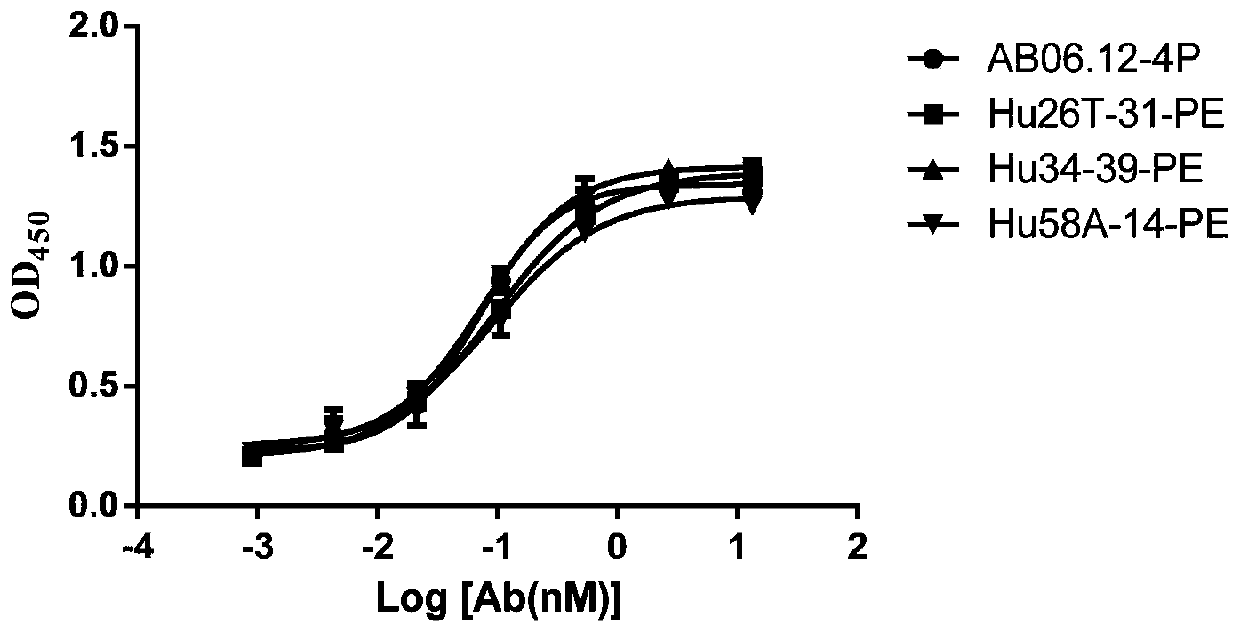
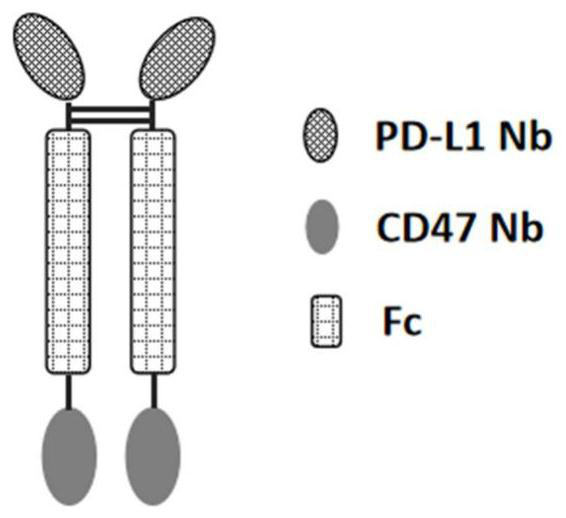
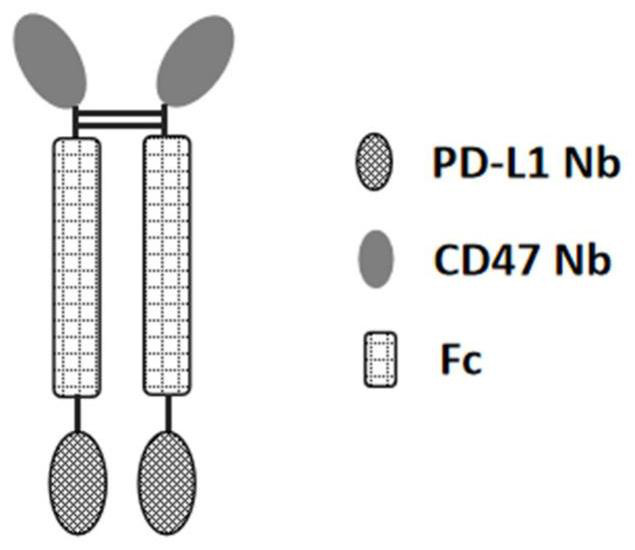
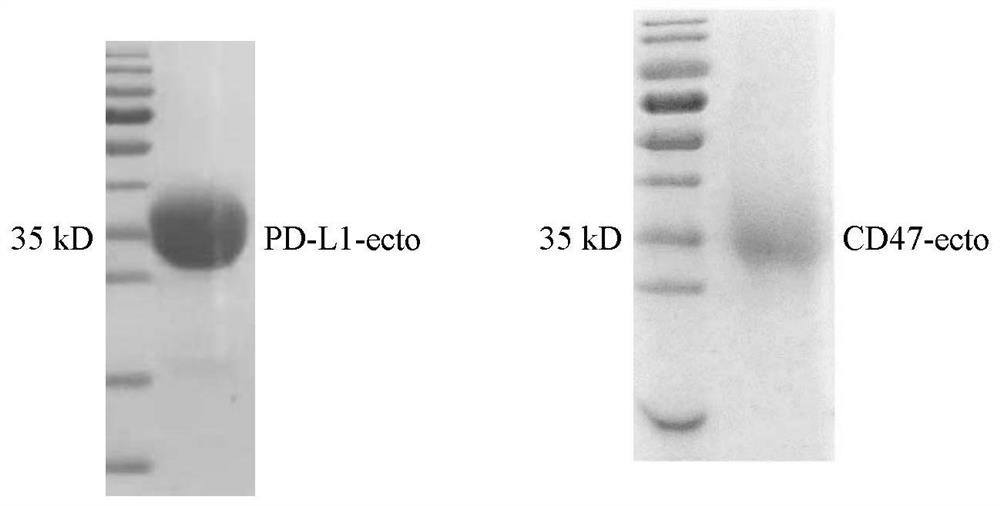
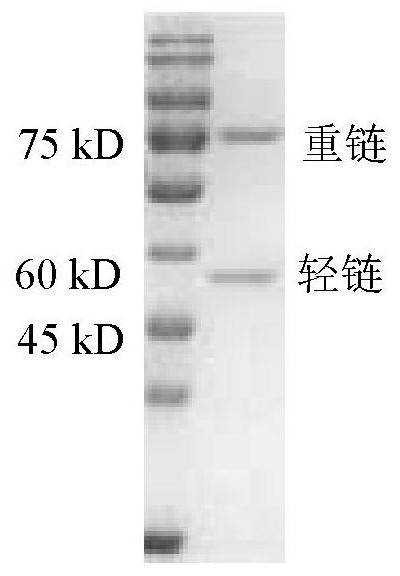
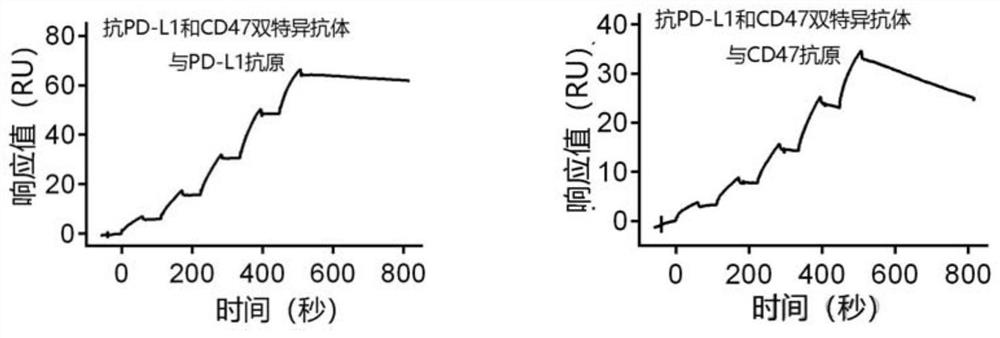
Anti-PD-L1/CD47 bispecific antibody and use thereof
ActiveCN112745392AHybrid immunoglobulinsAntibody mimetics/scaffoldsAntiendomysial antibodiesBispecific antibody
The invention provides an anti-PD-L1 / CD47 bispecific antibody and use thereof. Specifically, the invention provides a bispecific antibody which comprises (a) a PD-L1 single-domain antibody and (b) a CD47 single-domain antibody. The invention provides a coding sequence for coding the bispecific antibody, a corresponding expression vector, a host cell capable of expressing the bispecific antibody, and a production method of the bispecific antibody. The bispecific antibody can target PD-L1 and CD47 at the same time, can not only activate T cells, but also effectively promote phagocytosis of Jurkat cells by macrophages without causing human red blood cell agglutination, and has good application prospects.
Owner:SHANGHAI NOVAMAB BIOPHARM CO LTD
Method of detecting red cell antigen-antibody reactions
InactiveUS20080261247A1Reduce ionic strengthStabilize agglutinatesMaterial analysisCellular antigensLow ionic strength
A process for the detection of antibodies in a test sample by carrying out the following steps:(a) preparing an essentially isotonic and low ionic strength suspension comprising said test sample and erythrocytes;(b) incubating the erythrocytes, test sample and low ionic strength medium at 37-45° C. for various time periods to optimize antibody uptake;(c) combining said suspension with an amount of a solution of hexadimethrine bromide which is effective for agglutination of said erythrocytes and(d) separating the resultant agglutinates of polymer and erythrocytes from said suspension;(e) neutralizing the effect of hexadimethrine bromide by adding an effective amount of a gylcosaminoglycan in combination with other polybrene neutralizing agents;(f) monitoring the resuspended agglutinates for the presence or absence of antibody;(g) stabilizing the antibody dependent aggregate by adding an effective macromolecule as a component of the dispersing solution or separately and optionally.(h) packing and reincubating the test red cells to further increase the test sensitivity.
Owner:CLAVINA DIAGNOSTICS
Mesoporous bioactive glass/chitosan composite hemostatic sponge and preparation method thereof
ActiveCN112618781AGood biocompatibilityGood hemostatic effectSurgical adhesivesPharmaceutical delivery mechanismRed blood cellPolyvinyl alcohol
The invention discloses mesoporous bioactive glass / chitosan composite hemostatic sponge and a preparation method thereof. The method comprises the following steps of preparing mesoporous bioactive glass by using a bicontinuous microemulsion technology in combination with a sol-gel template method, blending a chitosan solution, a polyvinyl alcohol solution and the mesoporous bioactive glass, performing uniform dispersing, adding a cross-linking agent for a reaction, pouring a reaction product into a mold, performing pre-freezing and molding, and performing freeze-drying to obtain the mesoporous bioactive glass / chitosan composite hemostatic sponge. The composite sponge is simple in preparation process; red blood cells are agglutinated through the combination of polycations of a chitosan material and anions on the surface of a blood red cell membrane, platelet aggregation is activated, and thrombin is activated; the mechanical strength, porosity and water absorption rate of the sponge are improved due to the addition of the mesoporous bioactive glass; when being in contact with a wound, the sponge can rapidly absorb liquid, so that the aggregation of platelets and red blood cells is accelerated, blood clots are rapidly formed and accumulated in a sponge network structure with hierarchical pores, and finally the hemostatic effect is achieved; and the sponge has a good application prospect in the field of hemostatic materials.
Owner:SOUTH CHINA UNIV OF TECH +1
Kit for ABO blood type positive typing and Rh blood type detection as well as preparation method and detection method of kit
PendingCN111638373ARealize intuitive interpretationEasy to operateBiological testingRed blood cellBlood type positive
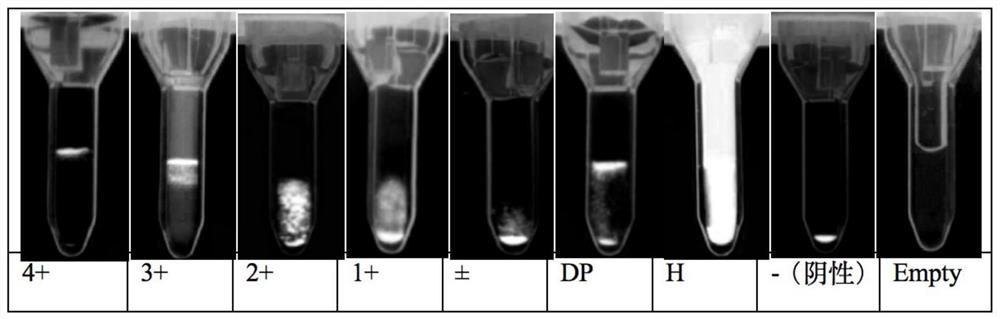
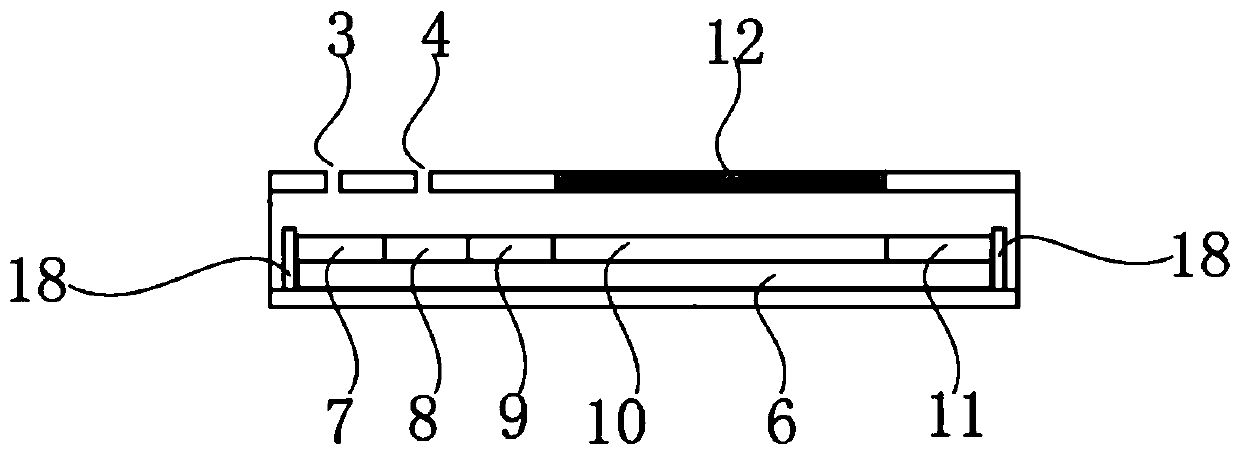
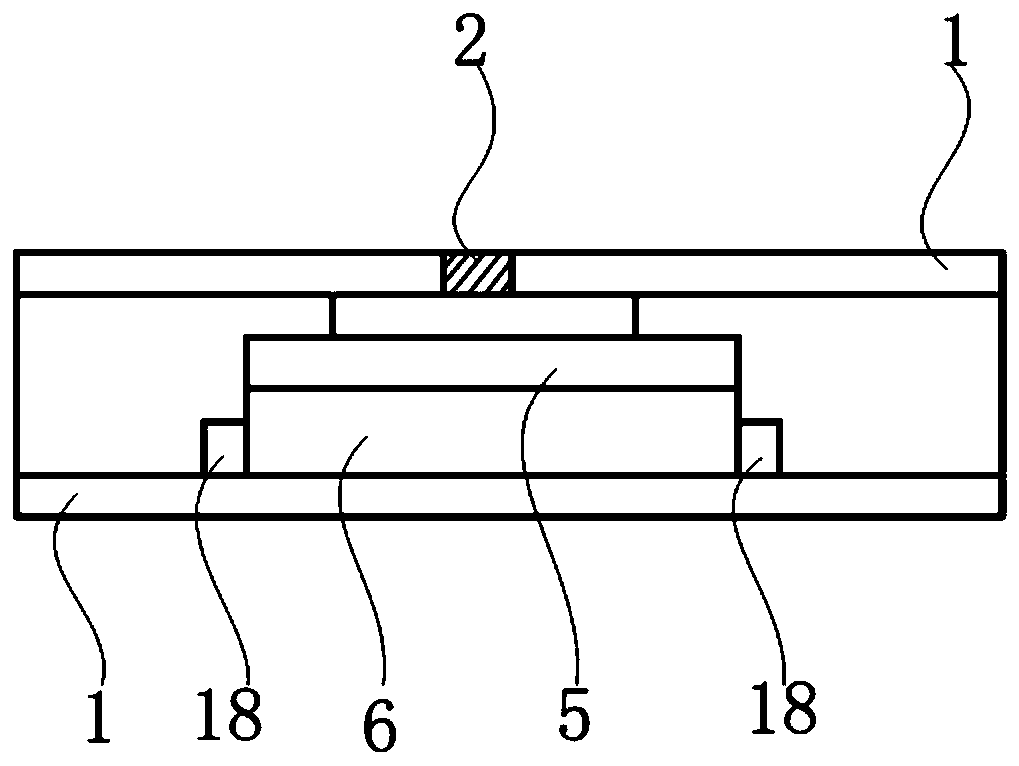
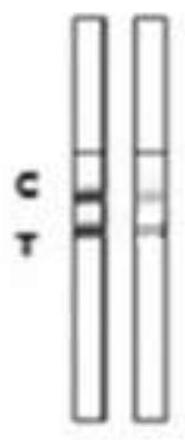
The invention discloses a kit for ABO blood type positive typing and Rh blood type detection as well as a preparation method and a detection method of the kit. The kit comprises a clamping shell whichis wrapped around an outer part, a detection film, a water absorption pad and a back lining that are located in the clamping shell and sequentially arranged from top to bottom. The detection film iscomposed of a character area and a non-character area, and the character area is composed of letters and symbols; the letters comprise letters A, B, O and letters at an Rh position; the letters at theRh position are one or more combinations of C, c, D, E and e; the symbols comprise a minus sign in the middle of the letter O and an plus sign on the upper right side of the letter Rh; each character comprises a hydrophilic area and a hydrophobic area, and the hydrophilic area is a reaction area. According to an immunofiltration principle, red blood cell agglutination is generated by utilizing specific reaction of red blood cell antigens and antibodies, whether red blood cell agglutination colors exist or not is observed, and a text consisting of colors is interpreted to realize blood type detection. The method is easy to operate, a detection result can be visually judged, and blood transfusion accidents caused by misjudgment are prevented.
Owner:长春博德生物技术有限责任公司
Sargassum thunbergii lectin and preparation method thereof
InactiveCN102690339AHigh activityPeptide preparation methodsAlgae/lichens peptidesIon exchangeGlycoprotein
The invention discloses a sargassum thunbergii lectin and a preparation method thereof, wherein the preparation method of the sargassum thunbergii lectin is characterized by selecting sargassum thunbergii as a raw material, carrying out lyophilization, pulverization, immersion, centrifugation, ammonium sulfate fractionation, DEAE-52 ion-exchange chromatography and SephadexG-200 gel filtration chromatography, thus producing the sargassum thunbergii lectin with a molecular weight of 17 kD. The sargassum thunbergii lectin of the invention can agglutinate a wide range of cells, has agglutinative functions for erythrocytes of rabbits, dogs, sheep, crucians, chickens and humans (A, B, AB), and the like, wherein minimum concentrations of agglutination reactions with rabbit and chicken erythrocytes are 4.4mg / ml and 0.55 mg / ml respectively, and shows hemagglutinin activity for rabbit erythrocytes even after heating treatment at 100 DEG C for 30 min (the activity decreases by 75%). A sugar inhibition experiment shows that the sargassum thunbergii lectin is only inhibited by glycoproteins as bovine thyroglobulin and gamma-globulin, and the minimum inhibition concentrations are 1.25 mg.mL<-1> and 2.5 mg.mL<-1> respectively.
Owner:DALIAN OCEAN UNIV +1
Influenza hemagglutination inhibition test detection method
PendingCN111323581ASolve the difficulty that the experiment cannot be carried outAvoid difficultiesBlood/immune system cellsBiological testingAntigenic analysisSpecific lectin
The invention belongs to the technical field of microbiological detection, and discloses an influenza hemagglutination inhibition test detection method which comprises the following steps: pretreatingstandard reference antiserum to remove non-specific inhibin and non-specific lectin in the serum; preparing an erythrocyte suspension; determining the hemagglutination titer of the influenza virus strain; preparing four hemagglutination unit antigens for an erythrocyte agglutination inhibition test; rechecking and titrating four hemagglutination unit antigens; and performing influenza virus identification and antigen analysis by using an HAI method to obtain the serum titer of the detected serum. According to the detection method, chicken erythrocyte, turkey erythrocyte and guinea pig erythrocyte are used as raw materials to prepare erythrocyte suspension, animal erythrocyte is used for replacing human erythrocyte, and the difficulty that an experiment cannot be conducted due to lack of erythrocyte is solved.
Owner:广州鸿泉生物科技有限公司
Method for agglutinating erythrocytes, method for separating erythrocytes, and hemagglutination reagent
Provided are: a method for agglutinating erythrocytes and a method for separating erythrocytes, wherein erythrocytes can be instantaneously agglutinated into a sufficient size from a blood sample, andthe erythrocytes can be completely separated from the blood sample; and a hemagglutination reagent. The method for agglutinating erythrocytes comprises adding a solution, containing a cholic acid-based surfactant and an acid, to a blood sample. The method for separating erythrocytes comprises separating the erythrocytes agglutinated by the method for agglutinating erythrocytes. In addition, the hemagglutination reagent contains a cholic acid-based surfactant and an acid.
Owner:DENKA CO LTD
Influenza virus attenuating method, influenza attenuated virus strain and application
PendingCN114381438APromote growthImprove growth characteristicsSsRNA viruses negative-senseViral antigen ingredientsBALB/cChick embryos
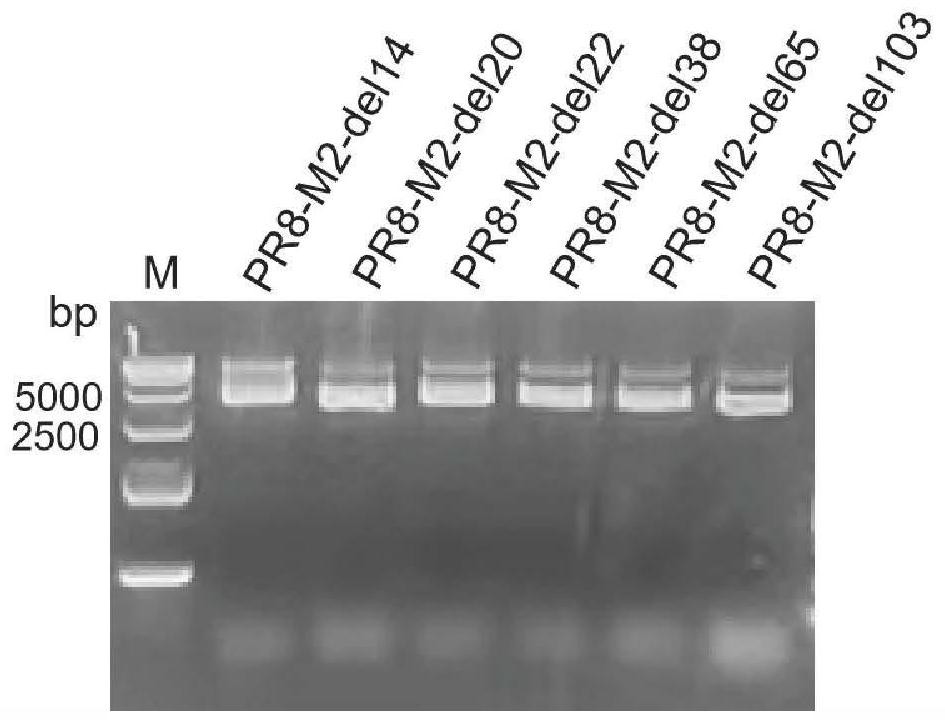
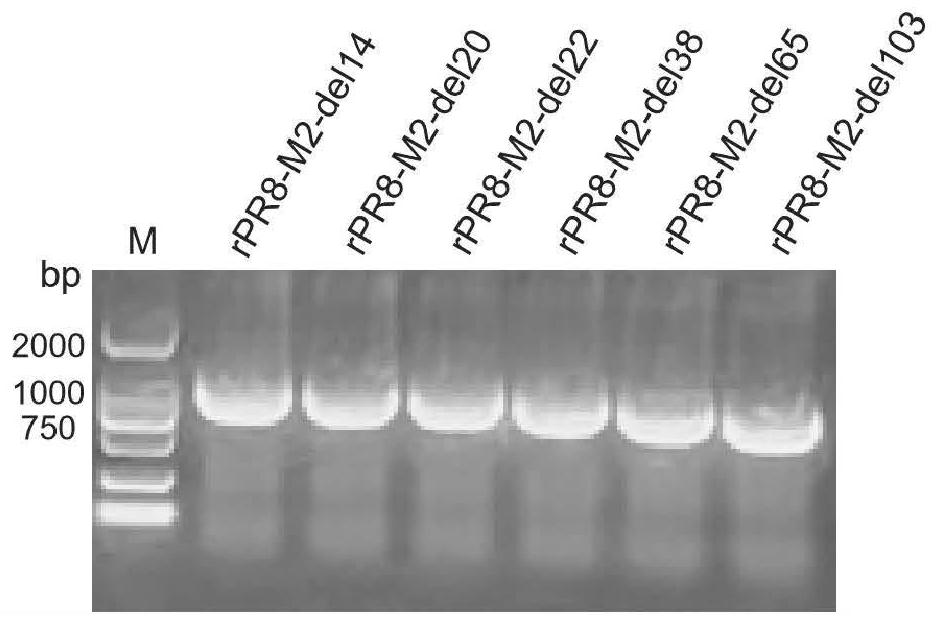
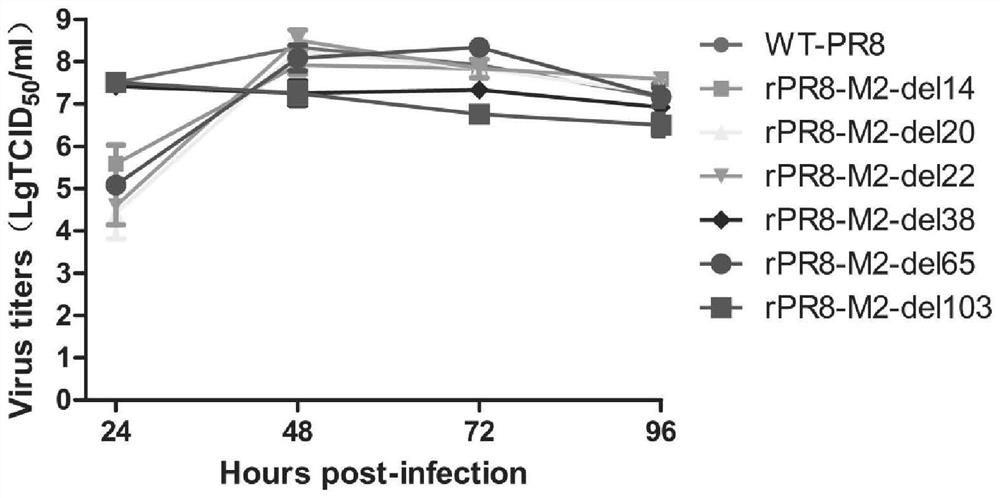
The invention relates to an influenza virus attenuating method, an influenza attenuated virus strain and application, and belongs to the technical field of biological medicine. The attenuating method comprises the following steps: carrying out random number and position base deletion on a transmembrane structural domain and a cytoplasm structural domain of M2 protein in an influenza virus conserved region, so as to obtain the influenza attenuated virus with corresponding base deletion. The influenza attenuated virus strain obtained by the attenuating method disclosed by the invention has a good growth characteristic on an MDCK cell line for expressing M2 protein; a high dose of virus strain can grow in MDCK cells or chick embryos and has a high chicken red blood cell agglutination price; in addition, a Balb / C mouse is immunized through nasal dripping, and it is found that the virus strain is non-pathogenic to the mouse relative to the parent virus IAV PR8. The virulence of the influenza virus is reduced in a random base deletion mode, and a foundation is laid for screening of safer and more effective IAV attenuated live vaccines.
Owner:ZHEJIAN DIFFERENCE BIOLOGICAL TECH CO LTD
Feature extraction method and device of blood type card image and blood type interpretation system
InactiveCN114332856AEasy to analyzeImprove interpretation accuracyAcquiring/recognising microscopic objectsBiological testingImaging processingImaging analysis
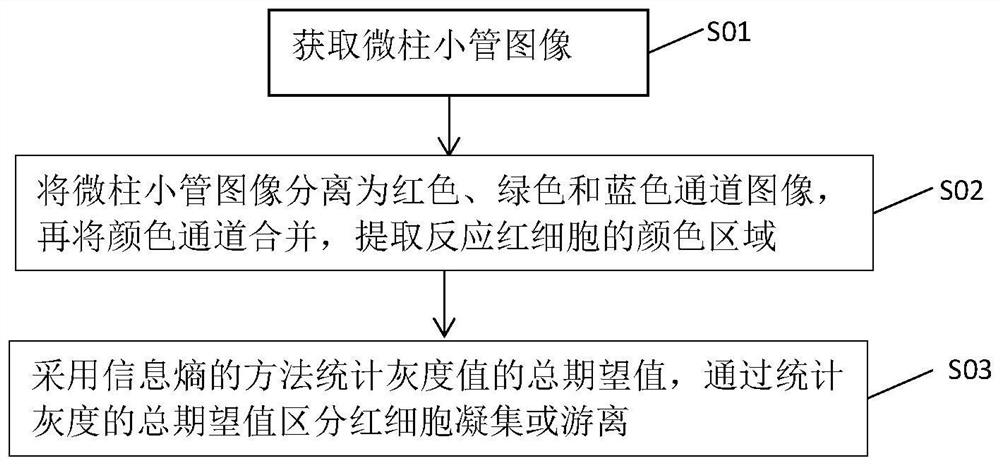
The invention belongs to the technical field of image processing, and discloses a blood type card image feature extraction method, which comprises the following steps: acquiring a microcolumn tubule image; separating the microcolumn tubule image into a red channel image, a green channel image and a blue channel image, merging color channels, and extracting a color region reflecting red blood cells; and counting the total expected value of the gray values by adopting an information entropy method, and distinguishing erythrocyte agglutination or dissociation by counting the total expected value of the gray values. Color features and gray features are comprehensively extracted, a novel feature extraction algorithm is provided, and the image analysis efficiency and interpretation accuracy of a blood type interpretation system are greatly improved.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
Compositions and methods for treating and/or preventing a urinary tract infection
InactiveUS20190000908A1Low effective doseAntibacterial agentsAntimycoticsAmerican cranberryUpper urinary tract infection
A composition comprising D-Mannose, Phellodendron Extract and Cranberry is disclosed. The disclosed composition, is useful in treating and preventing a microbial infection and induced red blood cells agglutination.
Owner:HMS LAB LTD
Indirect anti-human globulin accelerated blood matching test method
InactiveCN108548921AQuick checkoutSafe Blood Transfusion QualityMaterial analysisMatching testBlood plasma
The invention discloses an indirect anti-human globulin accelerated blood matching test method, which comprises: adding physiological saline to red blood cells, washing, uniformly mixing, carrying outcentrifugal separation, removing the supernatant, adding physiological saline, preparing a red blood cell suspension, adding plasma or serum, uniformly mixing, adding an indirect anti-human globulintest acceleration enhancer, uniformly mixing, incubating, adding physiological saline, carrying out centrifugal separation, slightly shaking the test tube, observing the result, and judging the resultaccording to the red blood cell agglutination or dispersion condition. According to the present invention, the cross matching test and / or the red blood cell irregular antibody screening test before the blood transfusion is accelerated and enhanced by using the indirect anti-human globulin test acceleration enhancer, and the indirect anti-human globulin test acceleration enhancer has characteristics of short incubation time, high sensitivity and strong accuracy, can be quickly detect various weak red blood cell irregular antibodies, and is suitable for emergency rapid cross-matching and red blood cell irregular antibody screening.
Owner:SECOND AFFILIATED HOSPITAL SECOND MILITARY MEDICAL UNIV
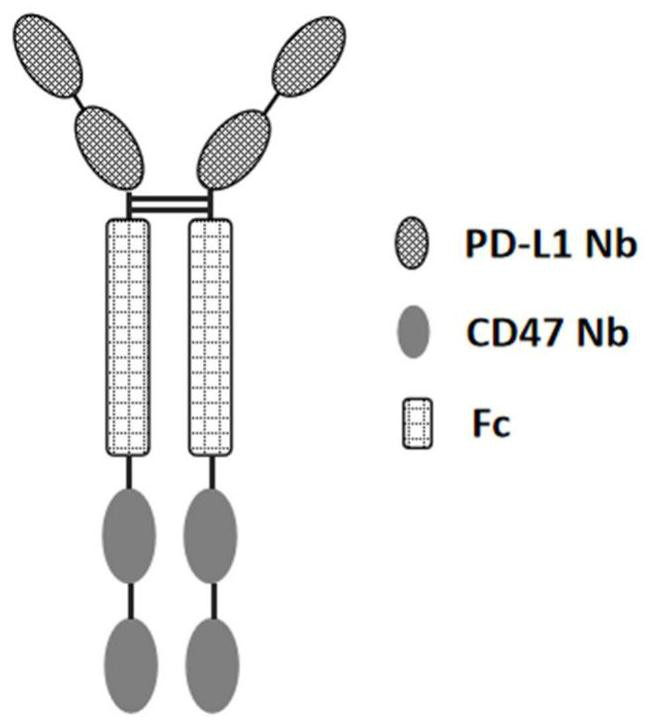
Non-erythrocyte agglutination anti-PD-L1/CD47 bispecific antibody and application thereof in anti-tumour treatment
ActiveCN113372449ADoes not cause agglutinationActivate phagocytosisHybrid immunoglobulinsAntibody ingredientsAntigenAntiendomysial antibodies
The invention relates to a non-erythrocyte agglutination anti-PD-L1 / CD47 bispecific antibody and application thereof in anti-tumour treatment. Specifically, the invention provides an anti-PD-L1 / CD47 bispecific antibody or an antigen binding fragment thereof; the anti-PD-L1 / CD47 bispecific antibody can be specifically bound with PD-L1 and CD47 molecules; binding of PD-L1 and PD-1 and binding of CD47 and SIRP alpha can be blocked at the same time after binding; T cells and macrophages are further activated to achieve the biological effects of resisting tumours and the like; and meanwhile, the antibody does not cause erythrocyte agglutination.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
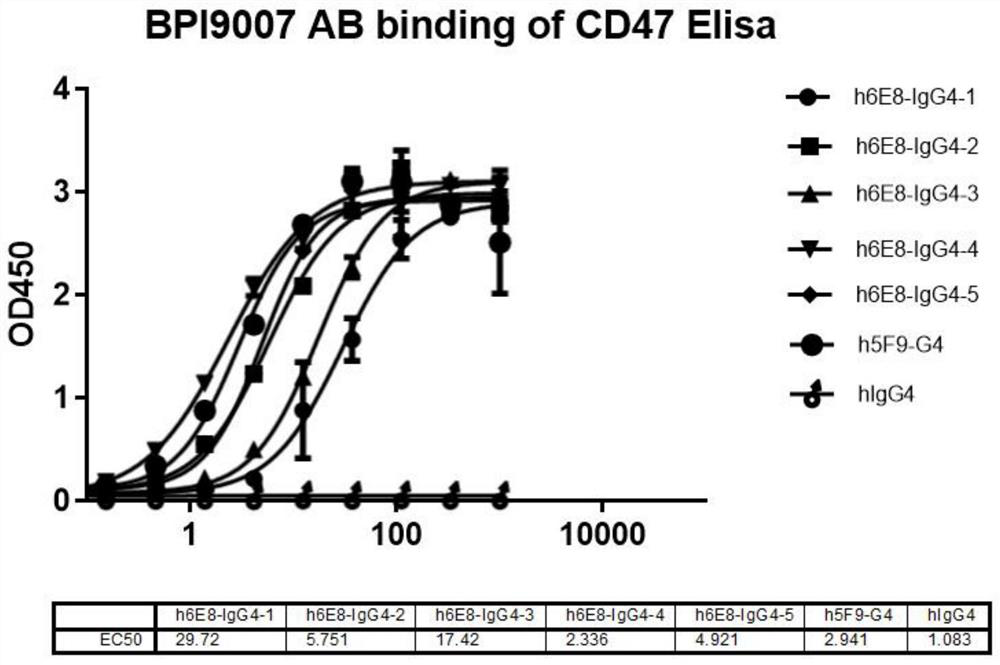
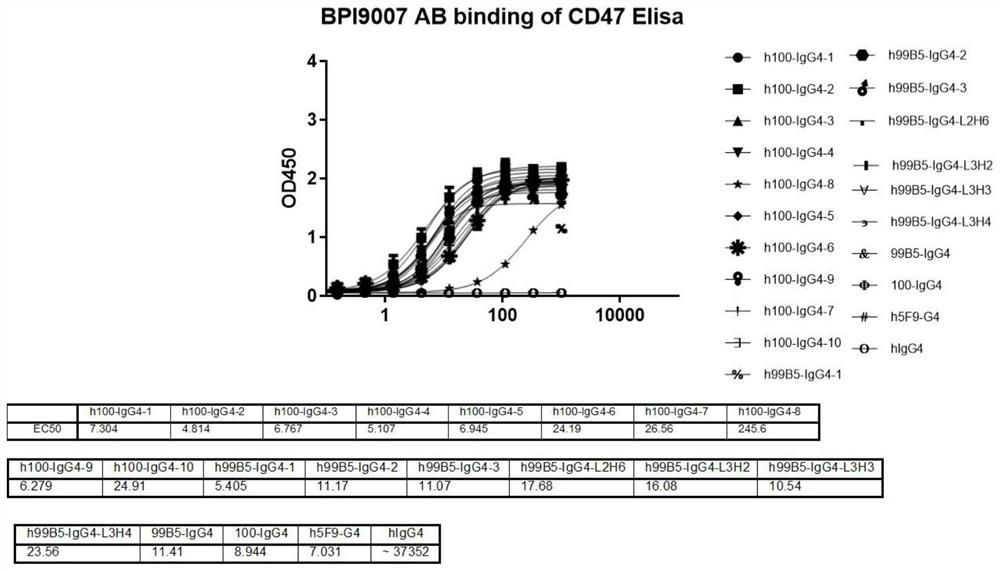
Humanized CD47 antibody or antigen binding fragment thereof and application
ActiveCN112679611ASpecific target specificityGrowth inhibitionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntiendomysial antibodies
The invention provides a humanized CD47 antibody or an antigen binding fragment thereof and application, the humanized CD47 antibody or the antigen binding fragment thereof is low in toxicity and efficient, erythrocyte agglutination does not occur in vitro, and erythrocyte removal cannot be caused. In addition, the humanized CD47 antibody provided by the invention shows extremely weak level of low binding or non-binding with platelets and red blood cells, and shows more specific targeting specificity on CD47+ tumor cells. The humanized CD47 antibody or the antigen binding fragment thereof provided by the invention can effectively block the binding of CD47 and SIRP alpha in function, and activate and mediate the phagocytic activity of macrophages to tumor cells.
Owner:BETA PHARM SUZHOU LTD
Sargassum thunbergii lectin and preparation method thereof
InactiveCN102690339BHigh activityPeptide preparation methodsAlgae/lichens peptidesAmmonium sulfate fractionationCentrifugation
The invention discloses a sargassum thunbergii lectin and a preparation method thereof, wherein the preparation method of the sargassum thunbergii lectin is characterized by selecting sargassum thunbergii as a raw material, carrying out lyophilization, pulverization, immersion, centrifugation, ammonium sulfate fractionation, DEAE-52 ion-exchange chromatography and SephadexG-200 gel filtration chromatography, thus producing the sargassum thunbergii lectin with a molecular weight of 17 kD. The sargassum thunbergii lectin of the invention can agglutinate a wide range of cells, has agglutinative functions for erythrocytes of rabbits, dogs, sheep, crucians, chickens and humans (A, B, AB), and the like, wherein minimum concentrations of agglutination reactions with rabbit and chicken erythrocytes are 4.4mg / ml and 0.55 mg / ml respectively, and shows hemagglutinin activity for rabbit erythrocytes even after heating treatment at 100 DEG C for 30 min (the activity decreases by 75%). A sugar inhibition experiment shows that the sargassum thunbergii lectin is only inhibited by glycoproteins as bovine thyroglobulin and gamma-globulin, and the minimum inhibition concentrations are 1.25 mg.mL<-1> and 2.5 mg.mL<-1> respectively.
Owner:DALIAN OCEAN UNIV +1
Method for wet detection of fingertip blood as well as kit and device thereof
ActiveCN107796809AAchieve separationAvoid breakingMaterial analysis by observing effect on chemical indicatorSedimentation analysisAnalyteMedicine
The invention belongs to the field of biochemical detection, and particularly relates to a method for wet detection of fingertip blood as well as a kit and a device thereof. According to the detectionmethod provided by the invention, wet detection of fingertip blood is carried out by adopting the method provided by the invention, and red blood cells in a fingertip blood sample are deposited by ared blood cell agglutination reagent in a first solution, so that the blood cells are rapidly condensed, serum and red blood cells are separated, and a target analyte is prevented from being interfered by blood cell rupture. The method comprises the following steps of: performing a second reaction between the fingertip blood sample diluted by the first solution and a second solution containing a hemoglobin detection reagent, detecting the hemoglobin concentration by a colorimetric method, and calculating a packed cell volume; and calculating a correction value of the concentration of the target analyte according to the obtained packed cell volume result. Therefore, the problem of deviation of a final test result caused by different packed cell volumes is avoided, and the accuracy of the test result is provided. The device and the kit for wet detection of fingertip blood, provided by the invention, are simple in structure and widely applicable to detection of various target objects.
Owner:SINOCARE
Immunochromatographic test strip for detecting object in red blood cell-containing sample and immunochromatography using the test strip
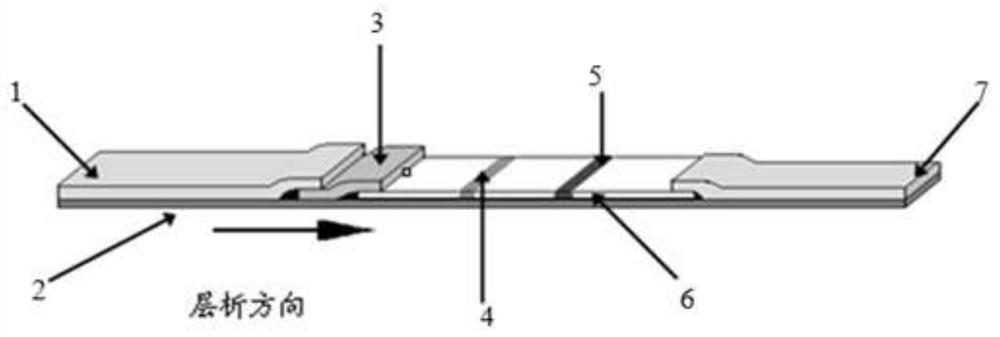
ActiveUS20200081002A1Prevent agglutinationAccurate detectionMaterial analysisRed blood cell sampleColloidal au
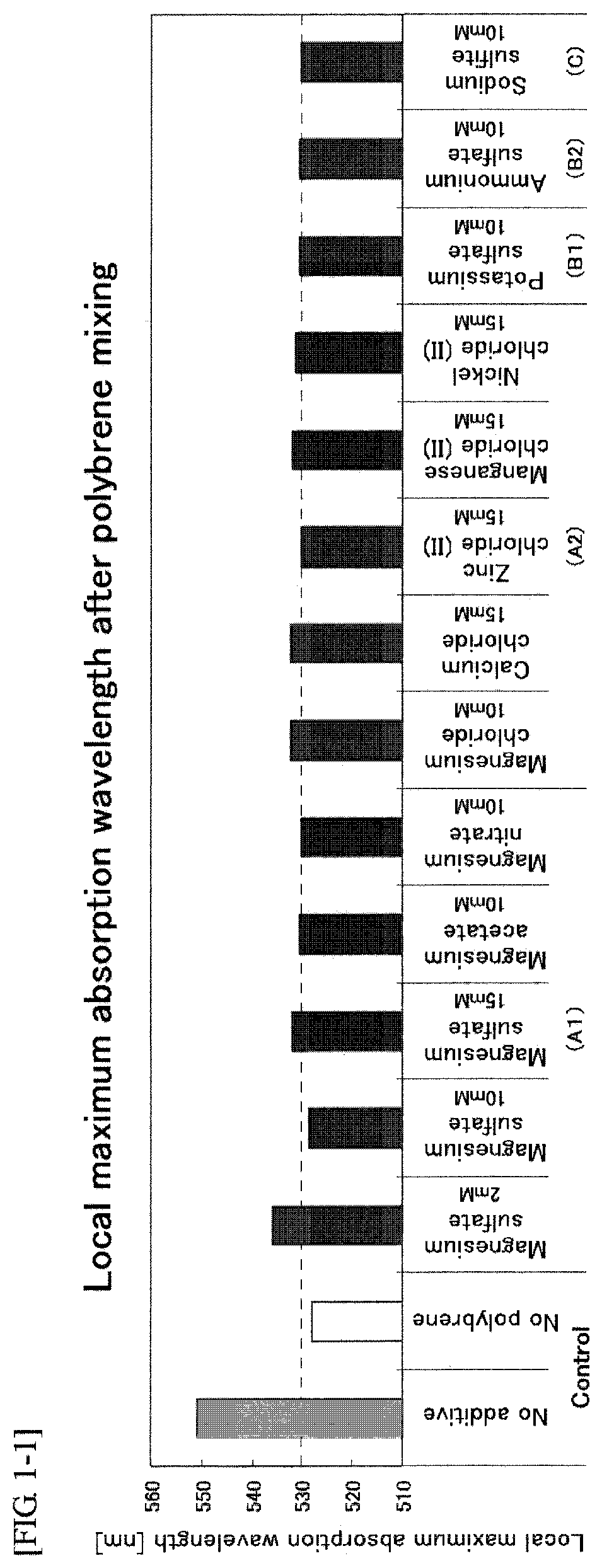
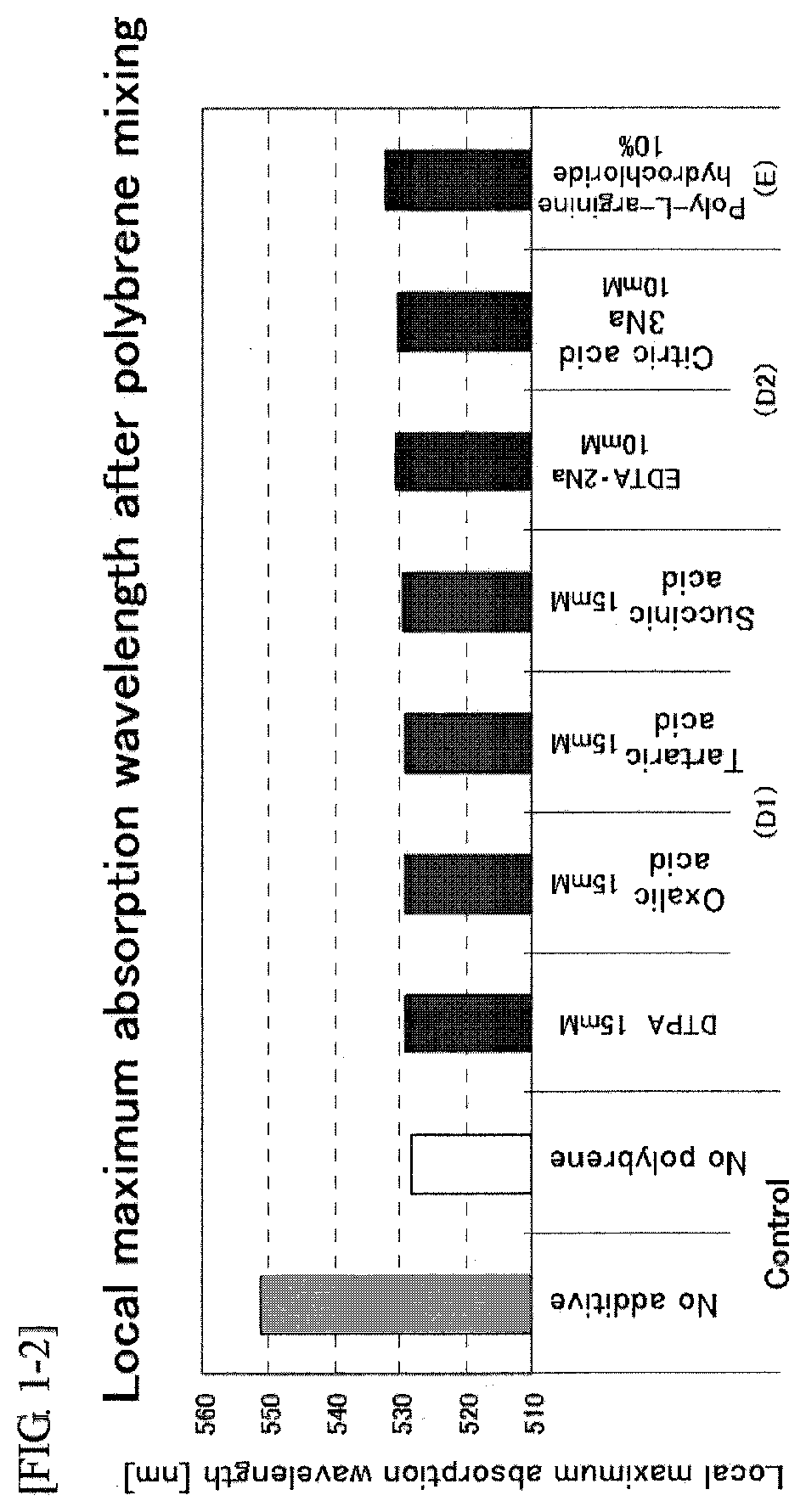
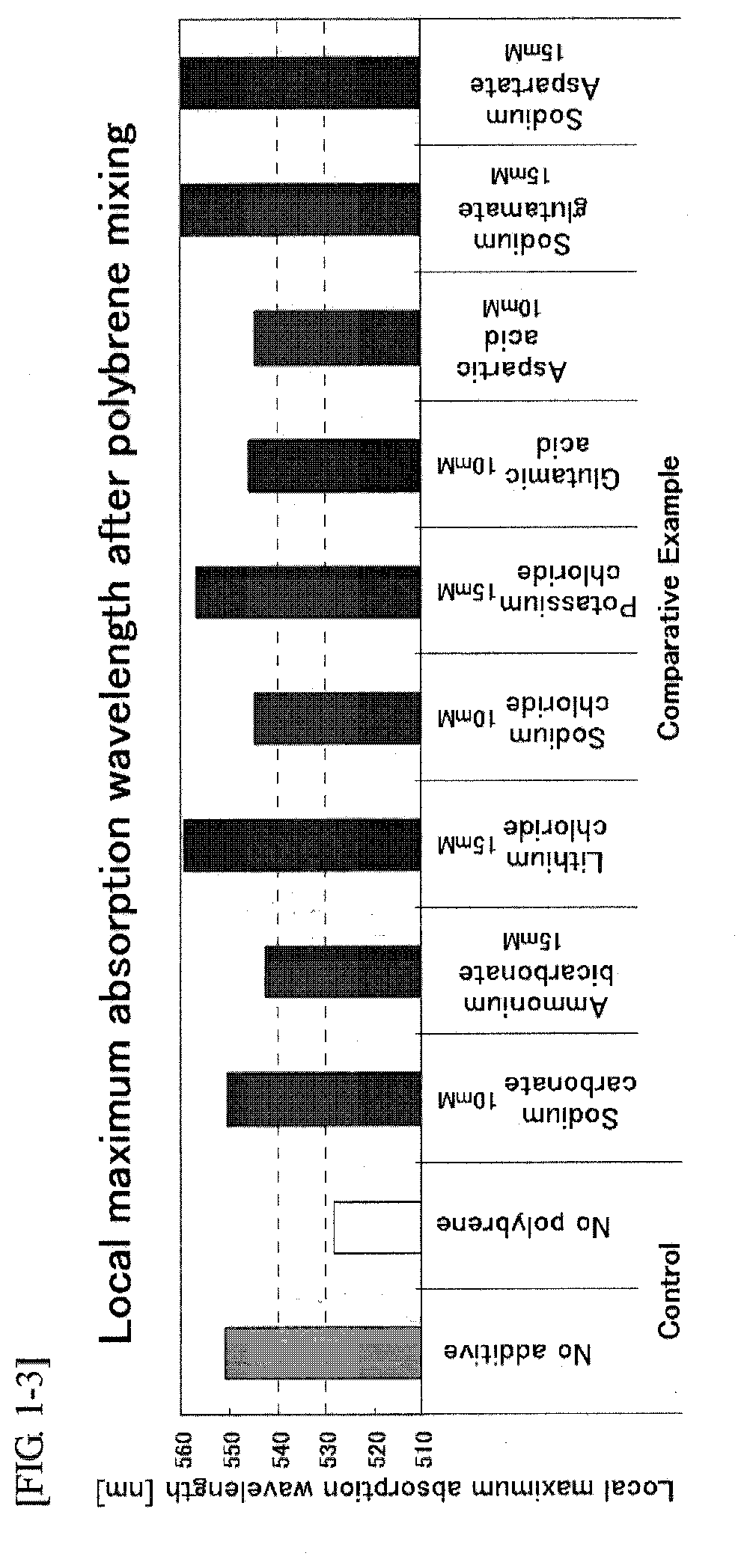
A problem of the present invention is to provide an immunochromatographic test strip avoiding agglutination of colloidal gold while red blood cells in whole blood are agglutinated and then separated and removed in the case of using polybrene as a hemagglutinating agent and the colloidal gold conjugates as a detection reagent, and to provide immunochromatography using the test strip. To solve the problem, the present inventors reviewed the composition of the existing reagent itself from a completely different viewpoint rather than the selection of type or amount of polyanions, and as a result of extensive study on each element, the inventors surprisingly found that agglutination of colloidal gold may be suppressed by using a particular additive without neutralization by polyanions.
Owner:SEKISUI MEDICAL CO LTD
Hybridoma cell strain 105D11, antibody and application thereof
ActiveCN110724672AStrong specificityHigh affinityBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntiendomysial antibodies
The invention discloses a hybridoma cell strain 105D11 and an anti-human CD47 monoclonal antibody generated from the hybridoma cell strain 105D11. A high-affinity anti-human CD47 specific monoclonal antibody which can block the interaction of CD47-SIRPalpha is prepared by using CD47 recombinant protein with bioactivity as an antigen through screening of a hybridoma technique. Meanwhile, an in-vitro experiment proves that the anti-human CD47 specific monoclonal antibody generated from the hybridoma cell strain 105D11 can promote macrophages to phagocytize tumor cells and cannot cause agglutination of erythrocytes. Therefore, the antibody has a potential value in tumor immunotherapy.
Owner:ZHEJIANG BLUE SHIELD PHARM CO LTD
Antioxidant Peptides and Their Genes of Frog Rana and Their Application in Pharmaceuticals
ActiveCN106432425BSimple structureEasy to synthesizeCosmetic preparationsNervous disorderCyclic peptideDisulfide bonding
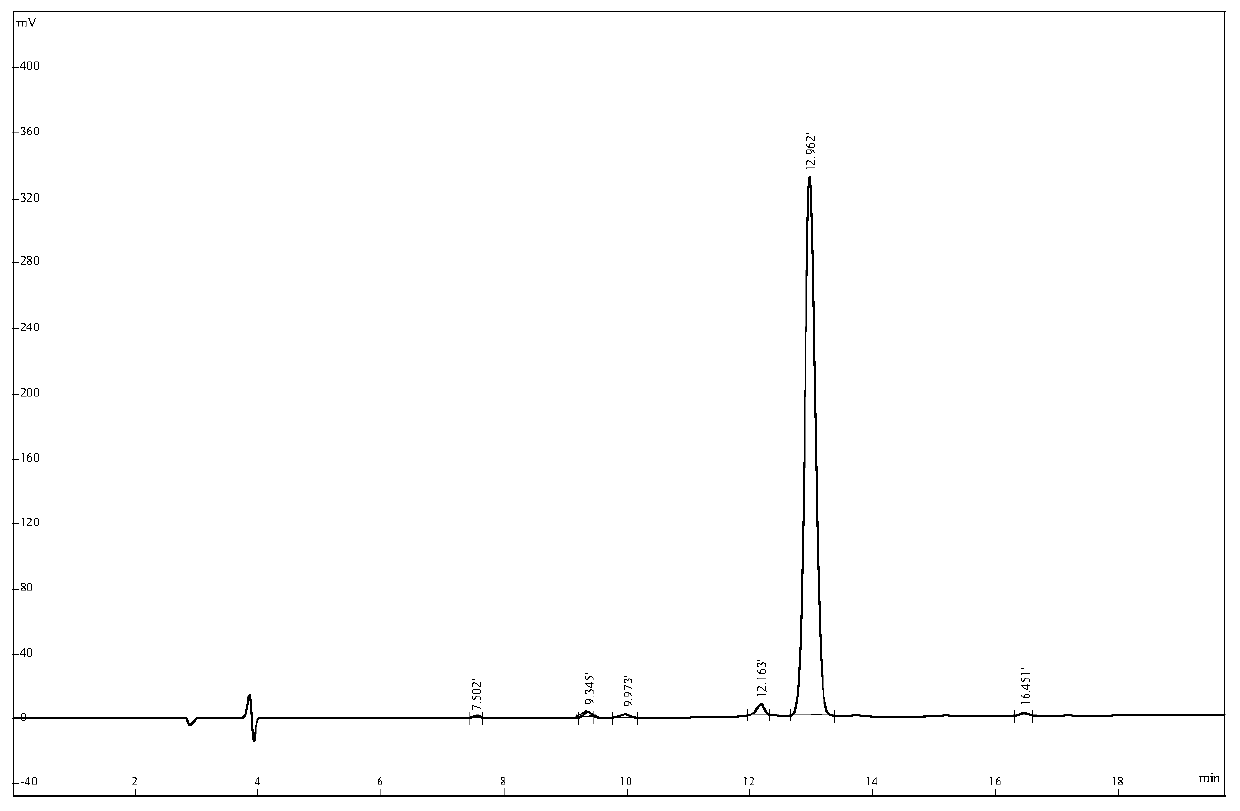
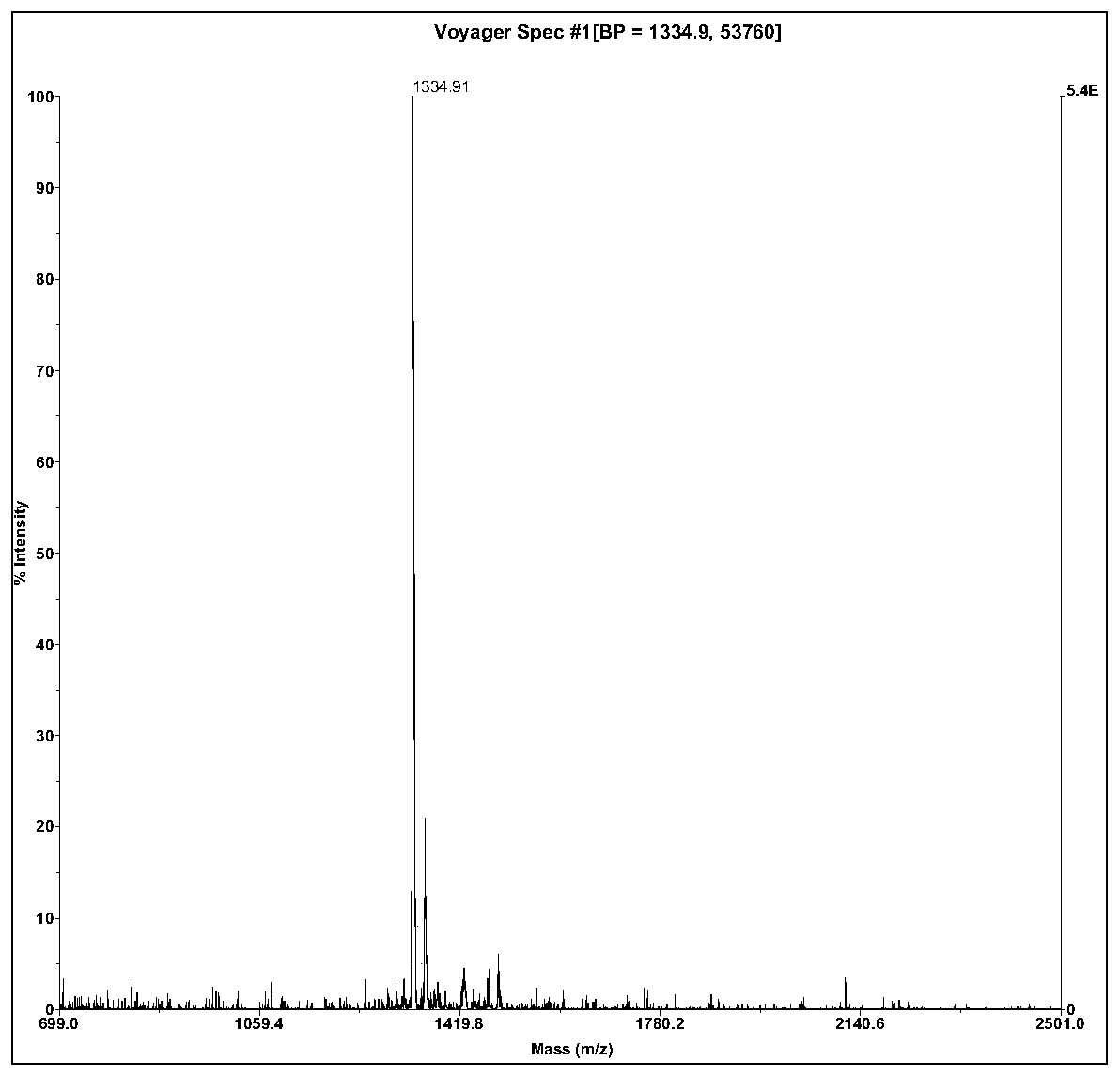
The invention relates to active polypeptide and a gene thereof, and application of the active polypeptide to pharmacy. Microhyla pulchra antioxidation peptide is cyclopeptide consisting of 12 pieces of amino acid, wherein the molecular weight is 1334.91 daltons; the isoelectric point is 8.002; the amino acid sequence is SEQ ID NO.1; and the third-position cysteine and the eleventh-position cysteine of the polypeptide form intramolecular disulfide bonds. The gene sequence of the microhyla pulchra antioxidation peptide consists of SEQ ID NO.4, and the gene encoded with the functional mature microhyla pulchra antioxidation peptide is the 187th to the 222nd position nucleotide. The gene of the microhyla pulchra antioxidation peptide deduces the mature functional polypeptide amino acid sequence, and the synthesized microhyla pulchra antioxidation peptide has strong erythrocyte agglutination and anti-oxidation functions.
Owner:SOUTHERN MEDICAL UNIVERSITY
Novel coronavirus COVID-2019 detection card and preparation method thereof
PendingCN111474352AImprove accuracyEasy to detectBiological material analysisAgainst vector-borne diseasesSpecific iggEngineering
The invention discloses a novel coronavirus COVID-2019 detection card and a preparation method thereof. The novel coronavirus COVID-2019 detection card comprises a card supporting layer attached to detection test paper, a sample unit positioned on the card supporting layer, a preprocessing unit positioned on the card supporting layer, a marker unit positioned on the card supporting layer, a detection unit positioned on the card supporting layer, and a recycling unit positioned on the card supporting layer; the sample units correspond to the sample holes; the pretreatment unit corresponds to the pretreatment hole; a hollow or transparent sealed detection window is arranged at the position, corresponding to the detection unit, of the shell; the erythrocyte agglutination layer is provided with erythrocyte agglutinin; the endogenous interfering substance treatment layer is provided with an endogenous interfering substance conjugate; the exogenous interfering substance treatment layer is provided with an exogenous interfering substance chelating agent; a non-specific IgM antibody treatment layer is provided with an IgM chelating agent; a non-specific IgG antibody treatment layer has anIgG precipitant. The problem that interfering substances influence the detection result of the novel coronavirus is solved, and the detection accuracy is improved.
Owner:北京乐普诊断科技股份有限公司
A colloidal gold detection card for rapid detection of rabbit plague virus and its preparation method
ActiveCN109856397BEffective controlAccurate detection of infectionMaterial analysisRabiesColloidal au
The invention relates to a colloidal gold detection card for rapidly detecting rabbit plague virus and a preparation method thereof. The specific steps are: (1) preparation of paired anti-rabbit plague virus monoclonal antibodies, including immune procedures, cell fusion, hybridoma screening and Cloning, antibody preparation and purification; (2) colloidal gold detection card for rapid detection of rabbit plague virus and its preparation method, including preparation of colloidal gold monoclonal antibody, sample pad treatment, spray film, C and T line determination, tested Sample processing, performance measurement. The invention can quickly, sensitively and accurately detect rabbit plague virus infection in the liver and kidney of rabbits, overcomes the domestic method of detecting rabbit plague virus mainly by red blood cell agglutination test, greatly shortens the detection time, and provides convenience for on-site detection.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
3F3Rmg recombinant fusion protein and preparation method thereof and application thereof to detection of dog rabies antibody
ActiveCN102492042AEasy to operateSimplify operating proceduresHybrid peptidesVector-based foreign material introductionRabiesRed cell agglutination
The invention discloses a 3F3Rmg recombinant fusion protein and a preparation method thereof. The recombinant fusion protein is encoded by a 3F3Rmg fusion gene obtained by splicing a 3F3ScFv gene and an Rmg gene, and has a base sequence shown in a sequence table SEQ.ID.No.1. Due to the difunctional characteristic of the 3F3Rmg recombinant fusion protein, the fusion protein can be combined with a human red blood cell without being agglutinated, can react with a rabies G antibody, and undergoes an agglutination phenomenon which can be observed by naked eyes under an antibody bridging action. A dog rabies antibody red cell agglutination test diagnostic reagent kit prepared by applying the recombinant fusion protein is easy to operate, has high sensitivity and high specificity, is particularly suitable for detecting the dog rabies antibody, and meets the requirements of use rapidness, easiness and convenience for a substrate field.
Owner:盐城市射阳荣港实业有限公司
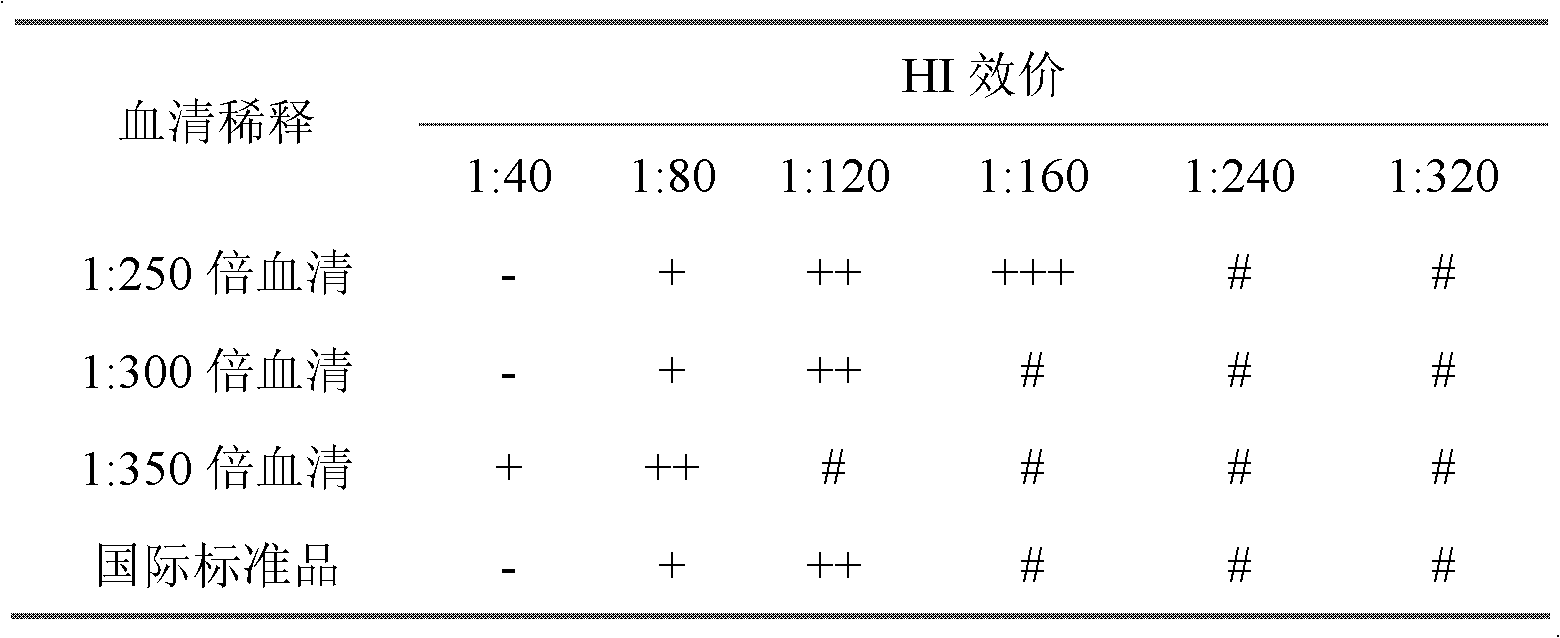
Positive serum national standard substance used for newcastle disease hemagglutination inhibition test and preparation method thereof
The invention relates to a positive serum national standard substance used for a newcastle disease hemagglutination inhibition test and a preparation method thereof. In the positive serum national standard substance used for the newcastle disease hemagglutination inhibition test, Newcastle Disease Virus (NDV) La Sota strain virus and inactivated vaccine immune SPF (Specific Pathoge Free) chicken are utilized to prepare hyperimmune serum to be used as a candidate, through the processes of candidate detection, standard substance preparation, detection, split charging, freeze-drying and the like, the positive serum international standard substance used for the newcastle disease hemagglutination inhibition test obtained by NIBSC (National Institute for Biological Standards and Control) is taken as a reference to carry out a red blood cell agglutination inhibition test to determinate a valence of the standard substance, and through a uniformity test, a stability test, cooperation calibration and data statistics, a constant valence value is ensured to be accurate, and the positive serum national standard substance used for the newcastle disease hemagglutination inhibition test is prepared. The method disclosed by the invention makes the standard substance preparation more scientific, rigorous and perfect according to the standard substance preparation process.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
CD47 targeting antibody and application thereof
PendingCN114656566AAgglutination does not causeActivate phagocytic activityBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesPlatelet
The invention relates to the field of biological medicine, in particular to a CD47 targeting antibody and application thereof. The anti-CD47 antibody provided by the invention can be specifically combined with tumor cells, blocks human SIRPa and human CD47 signals, can promote the phagocytosis of macrophages on the tumor cells, is high in affinity, strong in specificity and good in safety, does not cause red blood cell agglutination, and shows extremely weak combination with RBC and platelets.
Owner:GUANGDONG FEIPENG PHARM CO LTD
C-type lectin Nattectin gene of larimichthys crocea, C-type lectin Nattectin recombinant protein of larimichthys crocea and application of C-type lectin Nattectin recombinant protein
InactiveCN105061576AQuick clearHas agglutinating effectPeptide preparation methodsFermentationEscherichia coliBacteroides
The invention provides a C-type lectin Nattectin gene of larimichthys crocea, C-type lectin Nattectin recombinant protein of the larimichthys crocea and an application of the C-type lectin Nattectin recombinant protein, and relates to animal lectins. The amino acid sequence of C-type lectin Nattectin of the larimichthys crocea is represented as SEQ ID NO: 1. The nucleotide sequence of the C-type lectin Nattectin gene of larimichthys crocea is represented as SEQ ID NO: 2. A pET-32a-Nattectin recombinant expression vector is constructed; an escherichia coli expression strain pET-32a-Nattectin-BL21 with high-copy transformants is acquired; fermented cultivation and induced expression of the transformants are performed; recombinant protein Nattectin purification and renaturation are performed. The C-type lectin Nattectin recombinant protein of the larimichthys crocea can be applied to mediated bacteria agglutination and mediated red blood cell agglutination. The bacterial agglutination function and renaturation with one-step desalination method of Nattectin are discovered for the first time, a large amount of soluble bioactive protein is obtained finally, and the operation process is very simple.
Owner:JIMEI UNIV
Monoclonal antibody specifically binding to human CD47 and application thereof
PendingCN114539404AImprove anti-tumor efficacyHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMouse tumorErythroid cell
The invention provides a monoclonal antibody specifically binding to human CD47 or a fragment thereof, which can bind to CD47 on the surface of a cell and block binding of SIRP alpha to CD47 on the surface of the cell. The affinity KD value on the recombinant human CD47 is between 1 * 10 <-10 > and 8 * 10 <-8 >, and the tumor inhibition activity and the adverse reaction activity can be balanced. In the aspect of tumor inhibition activity, the antibody can inhibit tumor growth in a CD47 / SIRP alpha double-transgenic mouse tumor-bearing hCD47-MC38 subcutaneous transplanted tumor model, and can enhance in-vivo cell phagocytosis and prolong the lifetime of a mouse in a mouse leukemia model of transplanted human acute B lymphocytic leukemia; in the aspect of adverse reaction activity, the antibody does not have or only has reduced erythrocyte agglutination activity, and does not have a significant effect or only has an over-sexual effect on erythrocytes, platelets and hemoglobin.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com