Influenza virus attenuating method, influenza attenuated virus strain and application
A technology of influenza virus, virus strain, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Construction of Random Base Deletion Plasmid of Influenza Virus M2 Gene
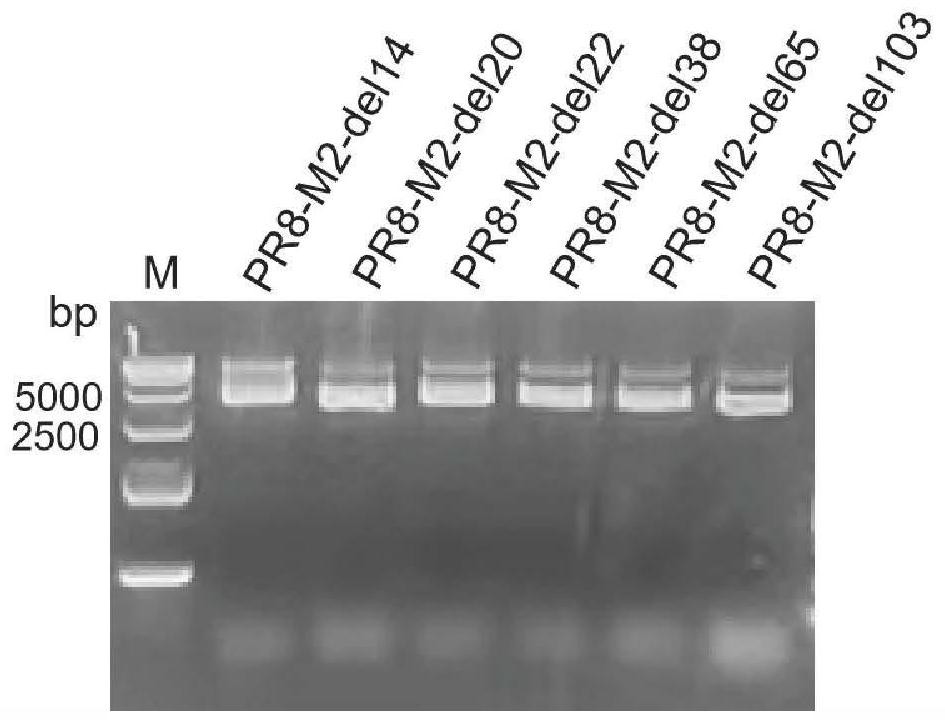
[0057] Extract the plasmid expressing the M2 gene of the influenza virus PR8 strain (the present invention has no special limitation on the construction method of this plasmid, adopt the commonly used plasmid and M gene known to those skilled in the art to carry out the construction of the plasmid overexpressing M2 according to the conventional recombinant plasmid construction method Just), using a series of base deletion primers (see Table 1 for the primer sequence) to carry out PCR amplification. After the correct molecular weight was identified by agarose gel electrophoresis, the target band was recovered and recombined at 50°C for 15 minutes, and the recombinant product was transformed into Escherichia coli competent cells. The extraction kit successfully prepared deletion plasmids PR8-M2-del14, PR8-M2-del20, PR8-M2-del22, PR8-M2-del38, PR8-M2-del65, PR8-M2-del103 (the extracted plasmid PR8- ...
Embodiment 2
[0061] Rescue and Validation of Replication-Restricted Influenza Viruses
[0062] HEK293T cells were plated in Thermo Fisher’s special six-well plate, and 12 hours later, plasmids containing 7 genes of PR8 (pFlu-PR8-PB2, pFlu-PR8-PB1, pFlu-PR8-PA, pFlu-PR8- NP, pFlu-PR8-NS, pFlu-PR8-HA, pFlu-PR8-NA), the M2 gene random base deletion series plasmids constructed in Example 1, and the plasmids expressing M2 protein were co-transfected into HEK293T cells. Change the medium 6-8 hours after transfection, freeze and thaw the cell plate once 48 hours after transfection, collect the supernatant and inoculate it into the T25 cell bottle of MDCK cells expressing M2 protein, observe the cell pathology after 72-96 hours of inoculation, and freeze the cell bottle After thawing once, the supernatant was collected by centrifugation and named rPR8-M2-del14, rPR8-M2-del20, rPR8-M2-del22, rPR8-M2-del38, rPR8-M2-del65, rPR8-M2-del103.
[0063] Utilize viral RNA extraction kit to extract the vira...
Embodiment 3
[0067] Replication-restricted influenza virus titer determination
[0068] Spread the MDCK cells expressing the M2 protein on a 96-well plate. After the cells have grown to a monolayer, take 50 μL of replication-limited influenza virus and add it to 450 μL of 1% TPCK opi-MEM, shake and mix well as system 1, and the final concentration is 10 -1 ; Take 50 μL from system 1 and add it to 450 μL 1% TPCK opi-MEM, shake and mix well as system 2, the final concentration is 10 -2 ; By analogy, the virus solution was diluted 10 times in a row and diluted to 10 times. -10 , then discard the medium in the 96-well plate, wash the plate with PBS, add 100 μL of the corresponding dilution of the virus solution to each well, do 3 repetitions for each gradient, and store at 37 ° C, 5% CO 2 After culturing for 72 hours in the cell incubator to observe the cell pathological changes, apply the Reed-Muench method to calculate its TCID 50 , virus titers are shown in Table 3.
[0069] Table 3 Sum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com