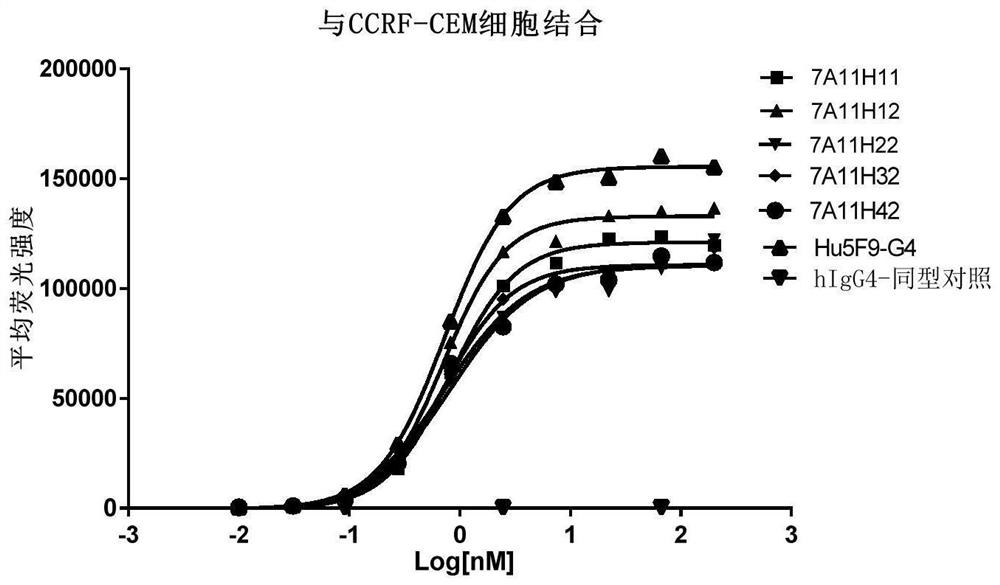
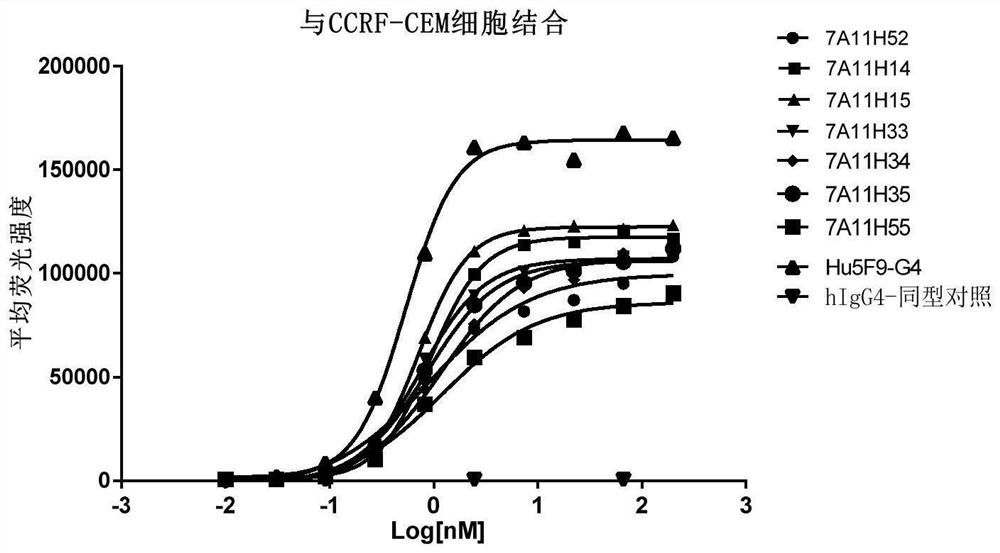
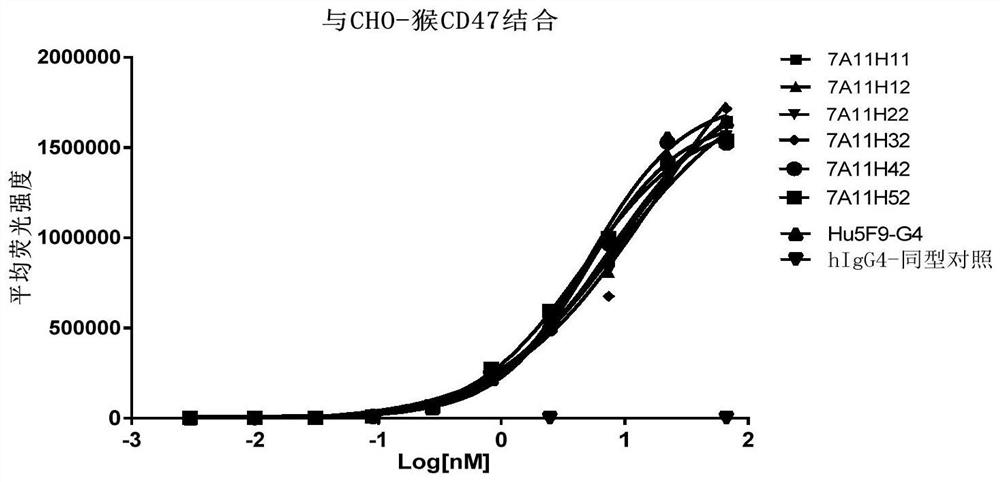
CD47 targeting antibody and application thereof
A technology of antibodies and antigens, applied in the fields of antibodies, applications, anti-tumor drugs, etc., can solve the problem that the inherent immune system has not been brought into play
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Screening of anti-CD47 mouse monoclonal antibody
[0070] 1. Construction of hCD47-ECD-HIS recombinant expression plasmid
[0071] Using the sequence provided by GenBank as a template (CEJ 95640.1), the full-length coding DNA sequence of the human CD47 extracellular region (hCD47-ECD) was synthesized and 6 HIS tag sequences were added at the 3' end, through the 5' end EcolI and 3' The terminal HindIII double restriction site was cloned into the expression vector pCDNA3.4 (Thermo Company) to establish the recombinant eukaryotic expression plasmid of CD47 extracellular full-length protein, namely hCD47-ECD-HIS recombinant plasmid DNA.
[0072] 2. Expression and purification of hCD47-ECD-HIS recombinant protein
[0073] Expression and purification of hCD47-ECD-HIS recombinant protein, including the following steps:
[0074] (1) The day before transient transfection, Expi293 (ThermoFisher Scientific; A14635) cells were passaged, and 2*10 cells were used in Dynam...
Embodiment 2
[0085] Example 2 Humanized design and combination of mouse monoclonal antibodies
[0086] The amino acid sequences of mouse antibody light chain CDRs are shown in SEQ ID NOs: 1-3:
[0087] 7A11D3D3-LCDR1 7A11D3D3-LCDR2 7A11D3D3-LCDR3 SEQ ID NO: 1 SEQ ID NO: 2 SEQ ID NO: 3 KSSQSLLNSRTRKNYLA WASTRES KQSYNLRT
[0088] The amino acid sequences of the CDRs of the mouse antibody heavy chain are shown in SEQ ID NOs: 10-12:
[0089] 7A11D3D3-HCDR1 7A11D3D3-HCDR2 7A11D3D3-HCDR3 SEQ ID NO: 10 SEQ ID NO: 11 SEQ ID NO: 12 SNWMN MIHPSDSETRLNQKFKD GTTVVDAFAY
[0090] The amino acid sequence of the murine antibody light chain variable region (>7A11D3D3-VL) is shown in SEQ ID NO:4.
[0091] The amino acid sequence of the murine antibody heavy chain variable region (>7A11D3D3-VH) is shown in SEQ ID NO:13.
[0092] Humanized design of mouse antibody, 7A11D3D3 mouse monoclonal antibody light chain design 5 humanized seque...
Embodiment 3
[0096] Example 3 Production of CD47 Antibody
[0097] The humanized variable domains were combined with secretion signals and human kappa and human FcIgG4S228P constant domains, cloned into a mammalian expression system, and transfected into 293 cells to generate humanized mAbs. Humanized variants were expressed as full-length IgG molecules, secreted into culture medium and purified using protein A.
[0098] Humanized antibody and positive control antibody Hu5F9-G4 (the sequence of Hu5F9-G4 has been disclosed in US Patent US2015 / 0183874A1) were transiently expressed and purified in Expi293 cells.
[0099] For transient expression of the antibody in Expi293 cells, vector pCDNA3.4 was used, and the heavy and light chains of the antibody were first cloned into separate pCDNA3.4 vectors, using PEI (purchased from Polysciences) chemical transfection reagent, following chemical transfection The pCDNA3.4 vector carrying the heavy and light chains of antibody molecules was transferre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com