C-type lectin Nattectin gene of larimichthys crocea, C-type lectin Nattectin recombinant protein of larimichthys crocea and application of C-type lectin Nattectin recombinant protein
A technology of recombinant protein and large yellow croaker, which is applied in the field of animal lectin, can solve the problems of bacterial agglutination, difficulty in maintaining biological activity, and difficult refolding of inclusion bodies without Nattectin, and achieve protein stability, easy binding, and simple refolding Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Acquisition of Nattectin cDNA Sequence of Large Yellow Croaker C Lectin
[0040] 1. Extraction of total RNA from the spleen of large yellow croaker and synthesis of cDNA by reverse transcription
[0041] The total RNA of large yellow croaker spleen was extracted with Trizol reagent, the integrity of RNA was detected by 1% agarose gel electrophoresis, and the concentration and purity of RNA were detected by ultraviolet spectrophotometry. The RNA was reversed with the M-MLV Reverse Transcriptase Kit to obtain the first strand of cDNA.
[0042] 2. Obtain the NattectincDNA sequence of large yellow croaker C lectin
[0043] The Nattectin gene sequence was spliced according to the large yellow croaker transcriptome database in our laboratory, and a pair of specific primers were designed to amplify the large yellow croaker C lectin Nattectin. The primer sequences are:
[0044] Forward primer NF: ATGGCATCAGCTCTTCATTTCA;
[0045] Reverse primer NR: TTGTAAGGCGTCCTTGGCAC.
...
Embodiment 2
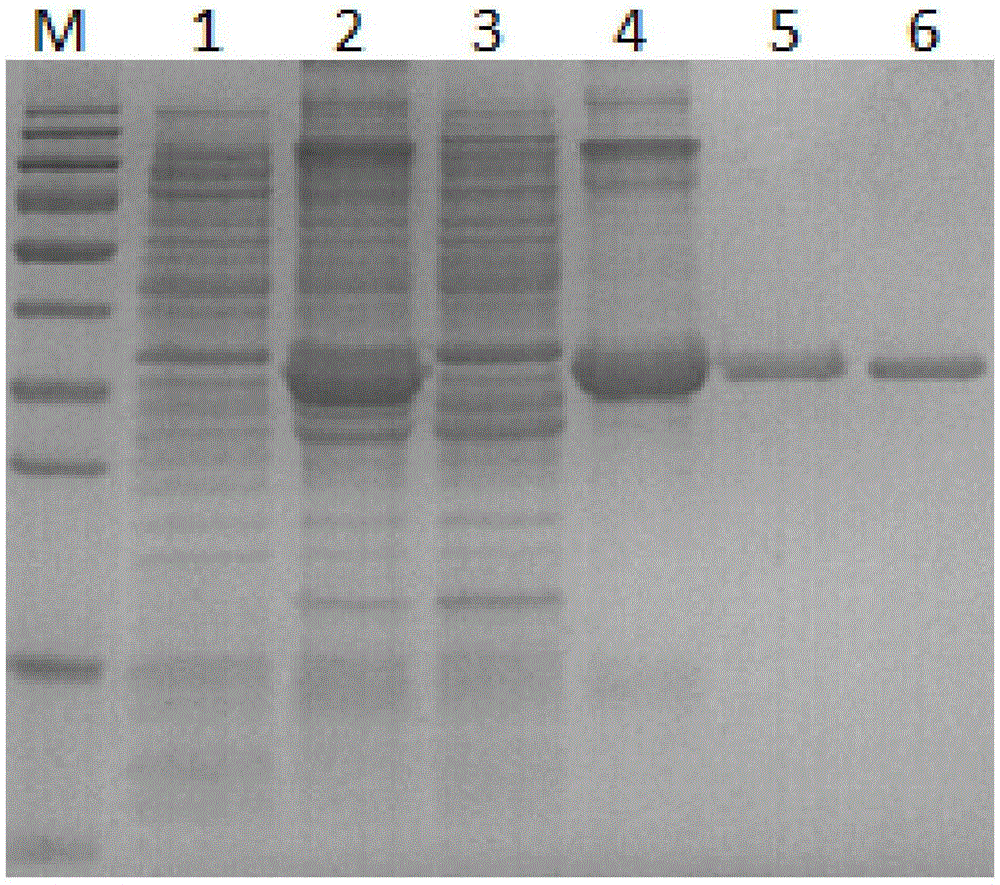
[0053] Preparation of Recombinant Protein of New C Lectin Nattectin from Large Yellow Croaker
[0054] 1. Construction of pET-32a-Nattectin expression vector
[0055] (1) PCR amplification of Nattectin gene
[0056] The total RNA of the spleen of large yellow croaker was extracted, and the cDNA of the spleen was amplified with OligdT as primer. According to the large yellow croaker transcriptome data (measured in our laboratory), the primers were designed as follows:
[0057] PF:CCG GAATTC ATGGCATCAGCTCTTCATTTCA (the underlined part is the restriction site of BamHI restriction); PR: CCG CTCGAG TTGTAAGGCGTCCTTGGCAC (the underlined part is the EcoRI restriction site).
[0058] Using the spleen cDNA of large yellow croaker as a template, the Nattectin gene of about 477 bp was amplified with primers PF / R.
[0059] (2) The PCR product and pET-32a were simultaneously digested with BamHI and EcoRI and recovered, and then ligated overnight at 16°C.
[0060] (3) After the ligati...
Embodiment 3
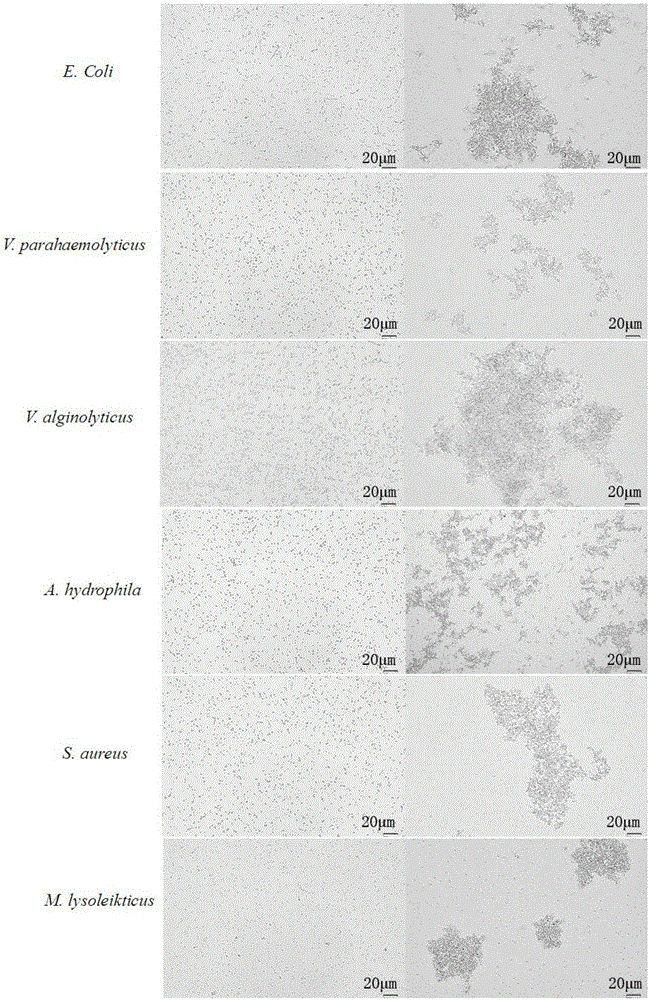
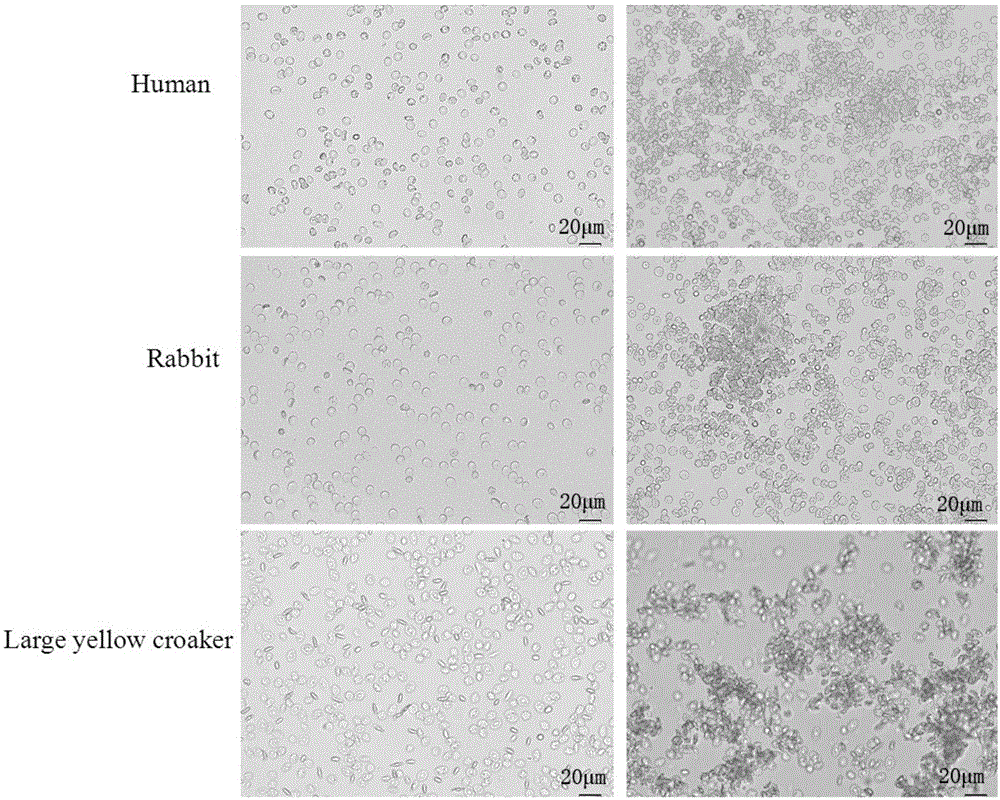
[0073] Agglutination of Recombinant Protein Nattectin on Bacteria
[0074] (1) Isolate and purify the bacteria, and adjust the cell concentration to 2.0×10 8 cell / ml.
[0075] (2) Bacterial agglutination was performed on a 96-well U-shaped plate. First add 50fμl TBS to each well after the first well, then add 100μl (150ug / mL) protein sample to the first well, mix well, then draw 50μl from the first well and add to the second well, and so on Doubling dilution. Finally, add various suspensions of bacteria to each well and mix thoroughly, and place at room temperature for 1 hour. Observe the agglutination status of various bacteria under a microscope and record the minimum agglutination concentration of Nattectin for different bacteria. At the same time, BSA was used as a negative control for parallel experiments.
[0076] (3) The results showed that the C lectin Nattectin recombinant protein was effective against Escherichia coli (E.Coli), Vibrio parahaemolyticus (V.parahaem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com