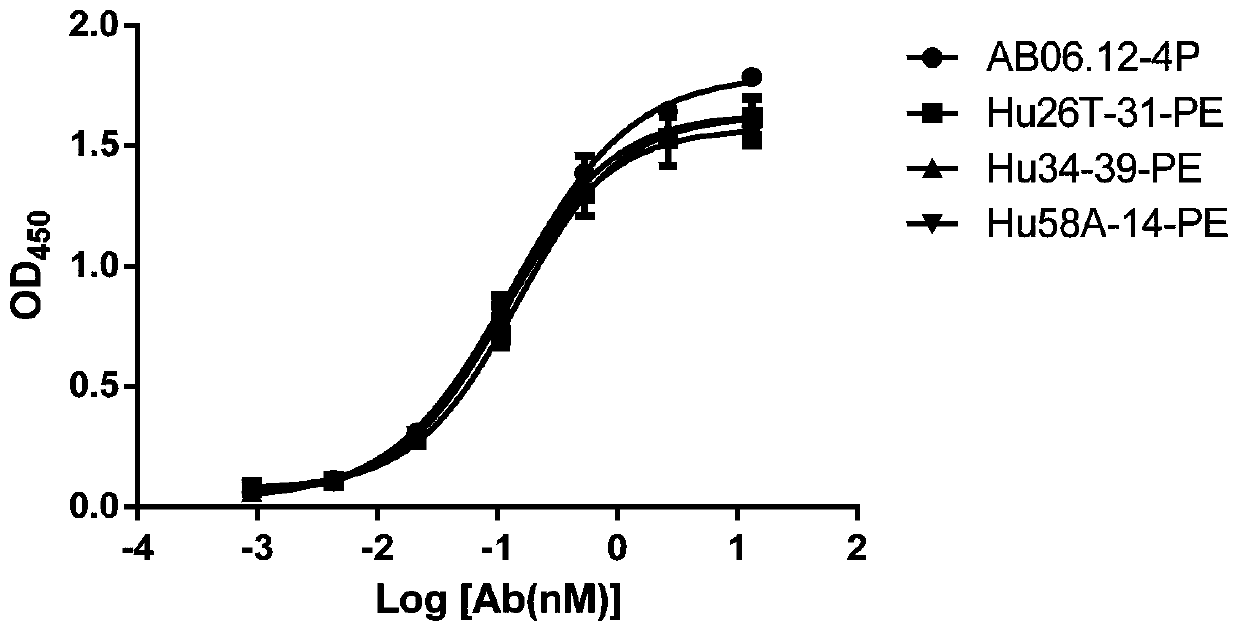
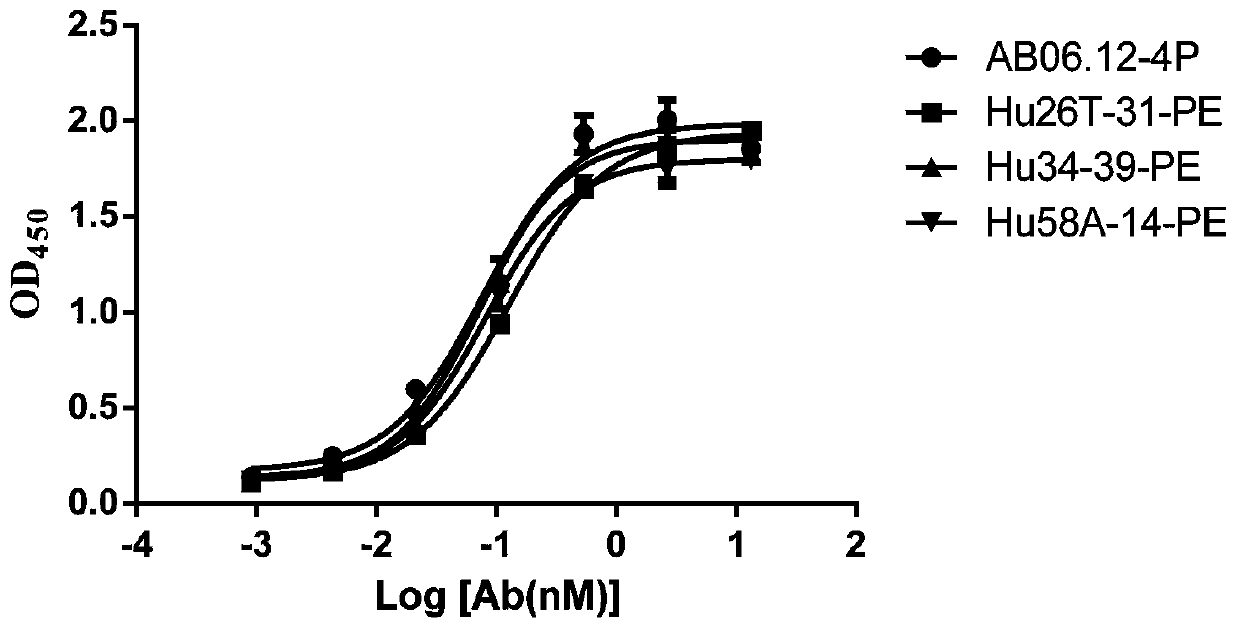
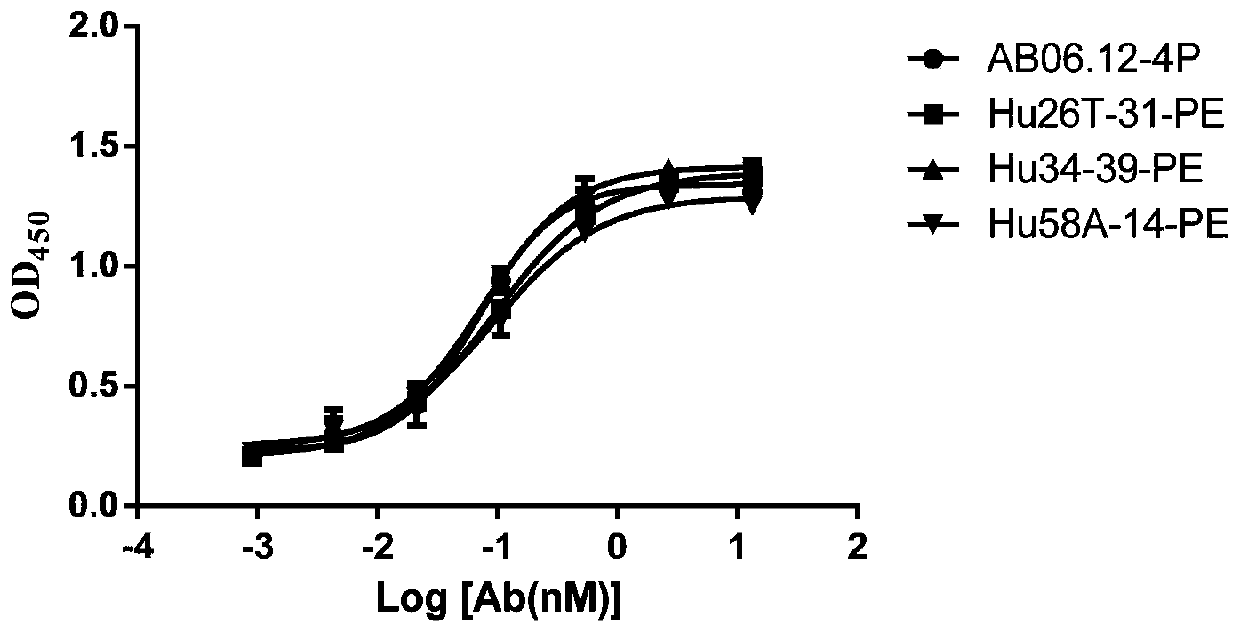
Anti-CD47 antibody and application thereof
A technology of antibody and humanized antibody, applied in application, antibody, anti-tumor drug, etc., can solve the problems of macrophage phagocytosis and cell loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] The preparation of embodiment 1 antigen protein and positive control antibody
[0099] 1. Construction of expression vectors for antigenic proteins and positive control antibodies
[0100] (1) Expression vector construction of antigenic protein
[0101] A gene fragment encoding the full-length CD47 protein was synthesized, and the amino acid sequence was designed as shown in SEQ ID NO: 41, and then cloned into the eukaryotic expression plasmid pTargeT to obtain its expression plasmid pTargeT-CD47.
[0102] The amino acid sequence of the extracellular region of human CD47 protein is fused with the amino acid sequence of hIgG1-Fc or his tag, and the amino acid sequence design is shown in SEQ ID NO:42 and SEQ ID NO:43 respectively. After codon optimization of the above amino acid sequence, the tagged CD47 protein extracellular region gene fragments CD47-hFc and CD47-his were synthesized, and cloned into the eukaryotic expression plasmid pHR respectively to obtain the expr...
Embodiment 2
[0129] The preparation of embodiment 2 monoclonal antibody
[0130] 1. Preparation of hybridoma monoclonal
[0131] (1) Animal immunity
[0132] The experimental mice of the SJL strain were immunized by co-immunization with anti-CD47 antigen proteins with different labels and adjuvants. The first antigen was 50ug of antigen, and the latter was immunized with 25ug of antigen.
[0133] The immune adjuvant can be Quick Antibody-Mouse5W (Beijing Boaolong Immunotechnology Co., Ltd.) or Titer Max (Sigma) and CpG (GenScript Biotechnology Limited Synthesis) / Alum (thermo) adjuvant spacer. The CD47 antigen protein samples with different labels were added dropwise to the adjuvant solution, and vortexed while dropping to mix thoroughly, and the dosage of the adjuvant was performed according to the instructions. After mixing evenly to form a water-in-oil emulsion, SJL mice were immunized.
[0134] Cell lines expressing high levels of CD47 such as CCRF-CEM and CHO-K1-E5 were also used to...
Embodiment 4
[0155] The construction of embodiment 4 chimeric antibody
[0156] Co-transform Escherichia coli DH5α with the purified mouse antibody light chain and heavy chain variable region gene fragments (see Example 1 for purification steps) and linearized eukaryotic expression plasmids containing human antibody light chain or heavy chain constant regions Competent cells, spread the mixture evenly on the surface of the agar plate containing the corresponding antibiotics, culture overnight in a constant temperature incubator at 37°C, pick several single colonies for DNA sequencing; mark the chimeric antibodies with correct sequencing as SHC025- 26CHI, SHC025-34CHI, SHC025-58CHI.
[0157] The positive clones with correct sequencing were inoculated in 2×YT liquid medium containing corresponding antibiotics, cultured with shaking at 37°C for more than 12 hours, and then the bacteria were collected for plasmid extraction to obtain chimeric antibody light chain and heavy chain expression pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com