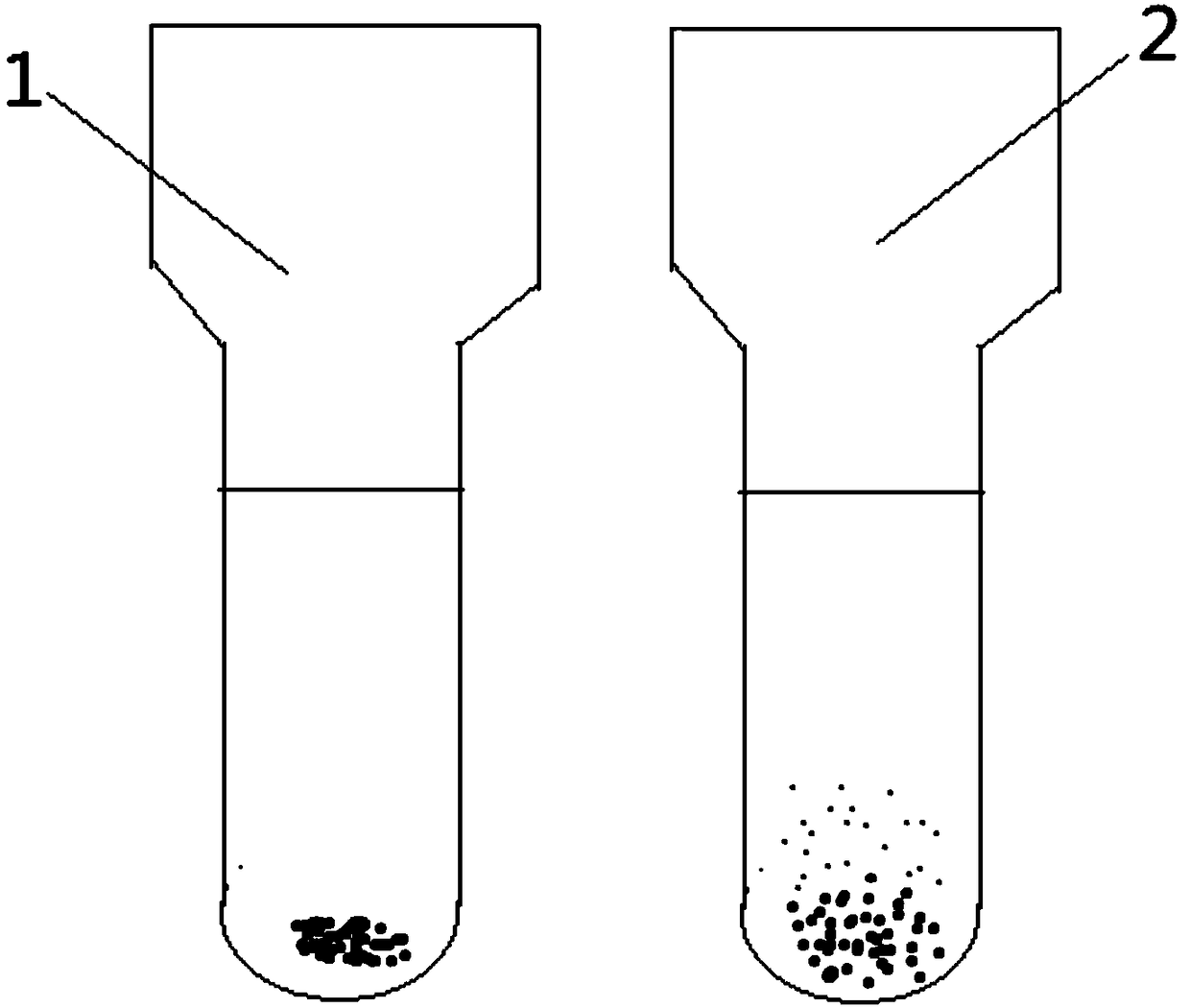
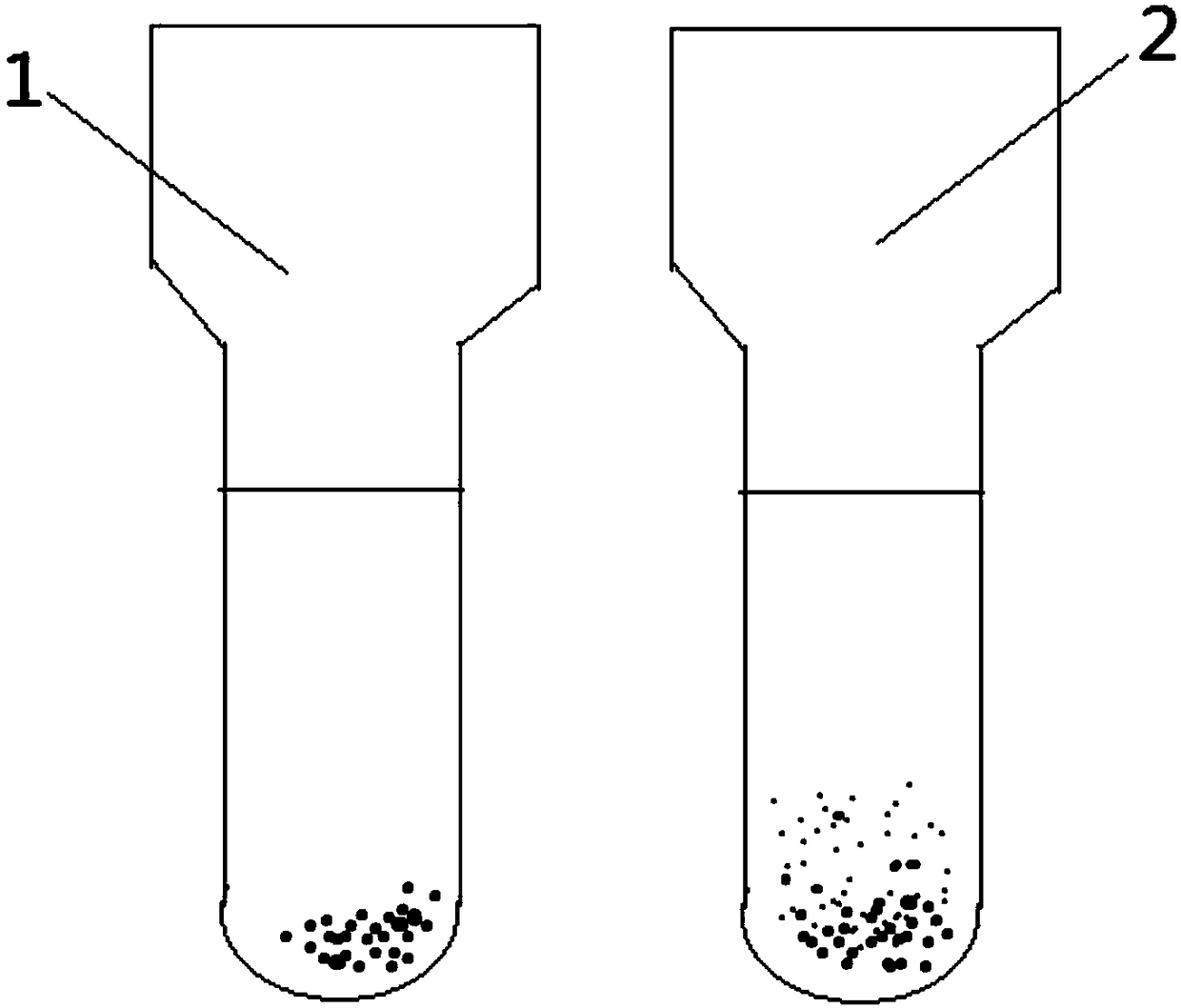
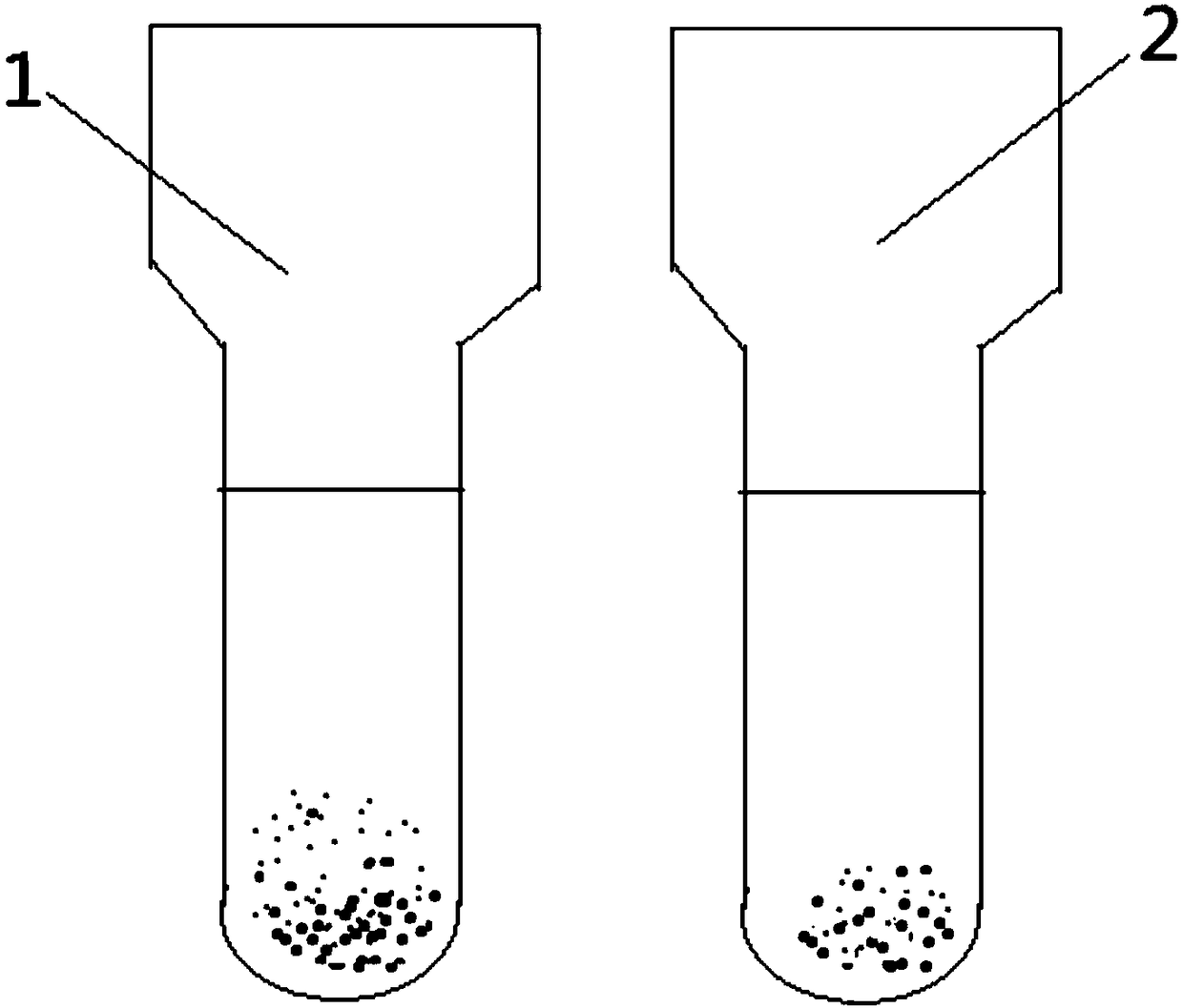
Indirect anti-human globulin accelerated blood matching test method
A technology of antihuman globulin and test method, which is applied in the field of indirect antihuman globulin accelerated blood matching, can solve the problems of low reaction sensitivity, adverse reactions of blood transfusion, long incubation time, etc., achieve high sensitivity, improve sensitivity and accuracy strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] An indirect antiglobulin test acceleration enhancer provided by the present invention comprises a polyethylene glycol (PEG) solution with a molecular weight of 2000 and a 0.5% polybrene solution with a concentration of 5%, said The volume ratio of polyethylene glycol solution and polybrene solution is 10:1.
[0077] The preparation method of described indirect antihuman globulin test accelerated enhancer, comprises the following steps:
[0078] Dissolve the polyethylene glycol powder in distilled water to make a 5% polyethylene glycol (PEG) solution, and dissolve the polybrene powder in distilled water to make a 0.5% polybrene solution , then the polybrene solution is added to the polyethylene glycol solution, the volume ratio of the polyethylene glycol solution and the polybrene solution is 10:1, and the pH value of the solution is adjusted to 7 with hydrochloric acid or sodium hydroxide to obtain the described Accelerated enhancer for indirect antiglobulin test. The...
Embodiment 2
[0080] An indirect antiglobulin test acceleration enhancer provided by the present invention comprises a polyethylene glycol (PEG) solution with a molecular weight of 8000 at a concentration of 20% and a polybrene solution at a concentration of 2.5%. The volume ratio of polyethylene glycol solution and polybrene solution is 20:1.
[0081] The preparation method of described indirect antihuman globulin test accelerated enhancer, comprises the following steps:
[0082] Dissolve the polyethylene glycol powder in distilled water to make a 20% polyethylene glycol (PEG) solution, and dissolve the polybrene powder in distilled water to make a 2.5% polybrene solution , then the polybrene solution is added to the polyethylene glycol solution, the volume ratio of the polyethylene glycol solution and the polybrene solution is 20:1, and the pH value of the solution is adjusted to 7 with hydrochloric acid or sodium hydroxide to obtain the described Accelerated enhancer for indirect antigl...
Embodiment 3
[0084] A kind of indirect antihuman globulin test accelerated enhancer provided by the present invention, comprises the polyethylene glycol (PEG) solution that concentration is 1%, molecular weight is 5000 and the concentration is the polybrene (polybrene) solution that concentration is 0.1%, described The volume ratio of polyethylene glycol solution and polybrene solution is 15:1.
[0085] The preparation method of described indirect antihuman globulin test accelerated enhancer, comprises the following steps:
[0086] Dissolve the polyethylene glycol powder in distilled water to make a 1% polyethylene glycol (PEG) solution, and dissolve the polybrene powder in distilled water to make a 0.1% polybrene solution , then the polybrene solution is added to the polyethylene glycol solution, the volume ratio of the polyethylene glycol solution and the polybrene solution is 15:1, and the pH value of the solution is adjusted to 7 with hydrochloric acid or sodium hydroxide to obtain the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com