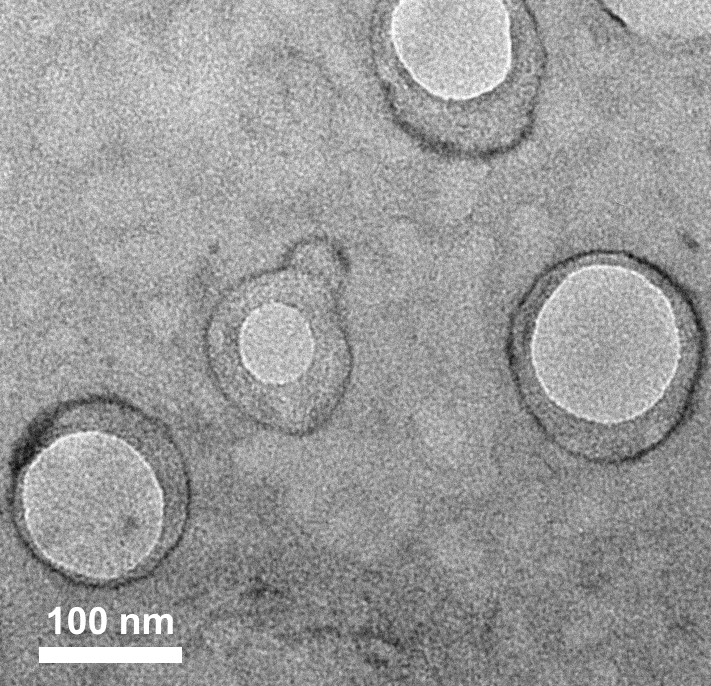
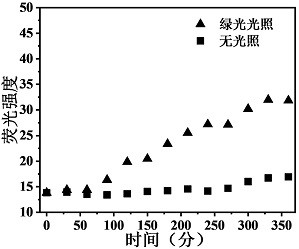
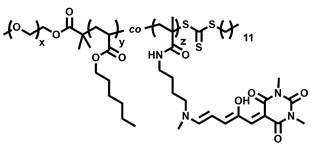
A green light-responsive polymer nano drug carrier
A nano-drug carrier and polymer technology, applied in the field of biomedical materials, can solve the problems of limiting the clinical application of polymer nano-drug carriers, and achieve the effects of avoiding light damage, large drug loading, and realizing release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of furan derivatives: Dissolve 1.51g of Michaelis acid or 1,3-dimethylbarbituric acid and 0.961g of furfural in 30mL of water, react at room temperature for 16h; after the reaction is completed, filter and wash with water, A yellow solid was obtained; the yellow solid was redissolved in dichloromethane, washed successively with saturated sodium bisulfite solution, ultrapure water, saturated sodium carbonate solution and saturated sodium chloride solution; finally dried over anhydrous magnesium sulfate, filtered and rotary evaporation treatment to obtain furan derivatives;
[0030] (2) Preparation of polyethylene glycol-b-poly(hexyl acrylate-co-pentafluorophenyl methacrylate): 1 part of 2-(dodecyltrithiocarbonate)-2-methyl Polyethylene glycol monomethyl ether propionate, 0.05 parts of azobisisobutyronitrile, 4 parts of pentafluorophenyl methacrylate, and 40 parts of hexyl acrylate were dissolved in 1 mL of dioxane, and reacted at 60 ° C for 2 h; After co...
Embodiment 2
[0035] (1) Preparation of furan derivatives: Dissolve 1.51g of Michaelis acid or 1,3-dimethylbarbituric acid and 0.961g of furfural in 30mL of water, and react at room temperature for 16h; after the reaction is completed, filter and wash with water. A yellow solid was obtained; the yellow solid was redissolved in dichloromethane, washed successively with saturated sodium bisulfite solution, ultrapure water, saturated sodium carbonate solution and saturated sodium chloride solution; finally dried over anhydrous magnesium sulfate, filtered and rotary evaporation treatment to obtain furan derivatives;
[0036](2) Preparation of polyethylene glycol-b-poly(hexyl acrylate-co-pentafluorophenyl methacrylate): 5 parts of 2-(dodecyltrithiocarbonate group)-2-methyl Polyethylene glycol monomethyl ether propionate, 0.1 part of azobisisobutyronitrile, 5 parts of pentafluorophenyl methacrylate, and 60 parts of hexyl acrylate were dissolved in 5 mL of dioxane, and reacted at 75 ° C for 15 h; ...
Embodiment 3
[0041] (1) Preparation of furan derivatives: Dissolve 1.51g of Michaelis acid or 1,3-dimethylbarbituric acid and 0.961g of furfural in 30mL of water, react at room temperature for 16h; after the reaction is completed, filter and wash with water, A yellow solid was obtained; the yellow solid was redissolved in dichloromethane, washed successively with saturated sodium bisulfite solution, ultrapure water, saturated sodium carbonate solution and saturated sodium chloride solution; finally dried over anhydrous magnesium sulfate, filtered and rotary evaporation treatment to obtain furan derivatives;
[0042] (2) Preparation of polyethylene glycol-b-poly(hexyl acrylate-co-pentafluorophenyl methacrylate): 10 parts of 2-(dodecyltrithiocarbonate group)-2-methyl Polyethylene glycol monomethyl ether propionate, 0.2 parts of azobisisobutyronitrile, 8 parts of pentafluorophenyl methacrylate, and 80 parts of hexyl acrylate were dissolved in 10 mL of dioxane, and reacted at 90 ° C for 30 h; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com