Electrochemical experiment method for rapidly evaluating corrosion resistance of coating
An experimental method and electrochemical technology, applied in the direction of weather resistance/light resistance/corrosion resistance, scientific instruments, measuring devices, etc., can solve problems such as unfavorable on-site rapid evaluation of coating systems, and achieve good application value and research significance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
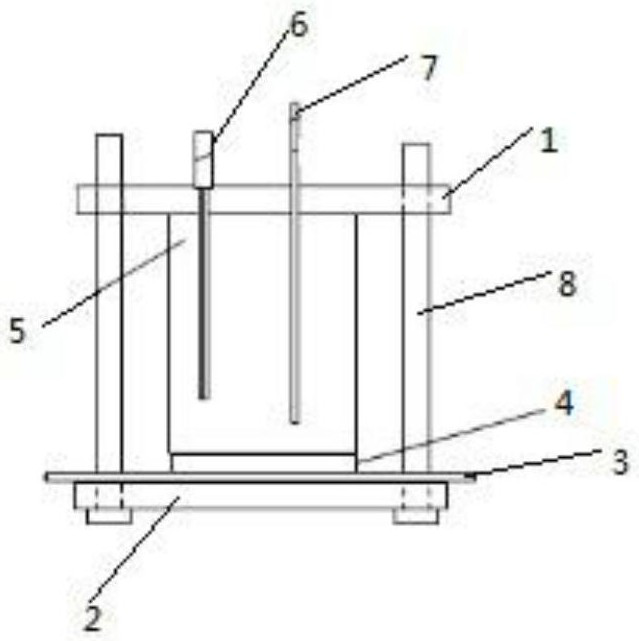
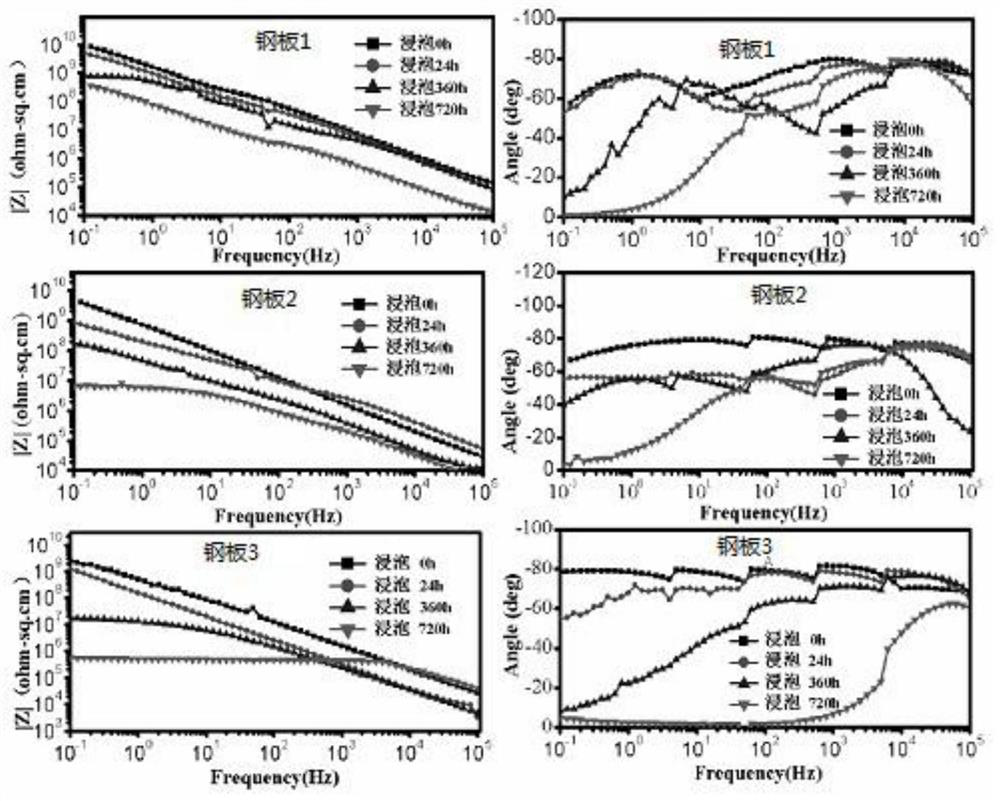
[0032] Soak the coated steel plate 1 in artificial seawater for 24 hours respectively, coat one side of the glass steel plate, and then install the steel plate in the electrochemical device, so that the side with the peeled coating is connected to the electrode of the electrochemical device. A proper amount of artificial seawater is injected into the device with a syringe, the connection between the electrochemical device, the electrochemical workstation and the computer is connected, and the entire electrochemical device is placed in a shielding box. Start the electrochemical workstation, which is the Princeton 273A electrochemical workstation. Before the test, set the impedance test parameters as follows:
[0033] DC potential: 0mV;
[0034] AC amplitude is 10mV;
[0035] Frequency scanning range: 100k-0.01HZ;
[0036] Logarithmic sweep, 10 points per decade;
[0037] The system was subjected to constant potential polarization, and the EIS was measured after the open cir...
Embodiment 2
[0039] Soak the coated steel plate 1 in artificial seawater for 360 hours, coat one side of the glass steel plate, then install the steel plate in the electrochemical device, and connect the side with the peeled coating to the electrode of the electrochemical device. A proper amount of artificial seawater is injected into the device with a syringe, the connection between the electrochemical device, the electrochemical workstation and the computer is connected, and the entire electrochemical device is placed in a shielding box. Start the electrochemical workstation, which is the Princeton 273A electrochemical workstation. Before the test, set the impedance test parameters as follows:
[0040] DC potential: 0mV;
[0041] AC amplitude is 10mV;
[0042] Frequency scanning range: 100k-0.01HZ;
[0043] Logarithmic sweep, 10 points per decade;
[0044] The system was subjected to constant potential polarization, and the EIS was measured after the open circuit potential was stable...
Embodiment 3
[0046]Soak the coated steel plate 1 in artificial seawater for 720 hours respectively, coat one side of the glass steel plate, and then install the steel plate in the electrochemical device, so that the side with the peeled coating is connected to the electrode of the electrochemical device. A proper amount of artificial seawater is injected into the device with a syringe, the connection between the electrochemical device, the electrochemical workstation and the computer is connected, and the entire electrochemical device is placed in a shielding box. Start the electrochemical workstation, which is the Princeton 273A electrochemical workstation. Before the test, set the impedance test parameters as follows:
[0047] DC potential: 0mV;
[0048] AC amplitude is 10mV;
[0049] Frequency scanning range: 100k-0.01HZ;
[0050] Logarithmic sweep, 10 points per decade;
[0051] The system was subjected to constant potential polarization, and the EIS was measured after the open cir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com