Artemisinin-encapsulated heme nanometer vesicle, preparation method and purpose
A technology of nanovesicles and artemisinin, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of lack of verification, improve encapsulation efficiency, increase target Effects of tropism, weak drug systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0058] Example 1: Synthesis and Characterization of Lipid-Hemin
[0059] 1, method:
[0060] 1-tattol-SN-propanediol-phosphate (Lyso 16:0) added to 50 ml round bottom flask, dissolved with anhydrous dichloromethane, add 0.5 equivalent hemin, 0.75 equivalent 4-two Metaminopyridine (DMAP), 3 equivalents of 1- (3-dimethylaminopropyl) -3-ethyl carbon diimide (EDC), if necessary, 1 ml N, N-dimethylformamide ( DMF) Help. The mixture was stirred for 48 hours under anhydrous oxygen-free dark conditions to complete the esterification reaction. Dry repeatedly. Further, the purified lipid-hemin was obtained by the column chromatography chromatography.
[0061] The reaction steps are as follows:
[0062]
[0063] 2, result
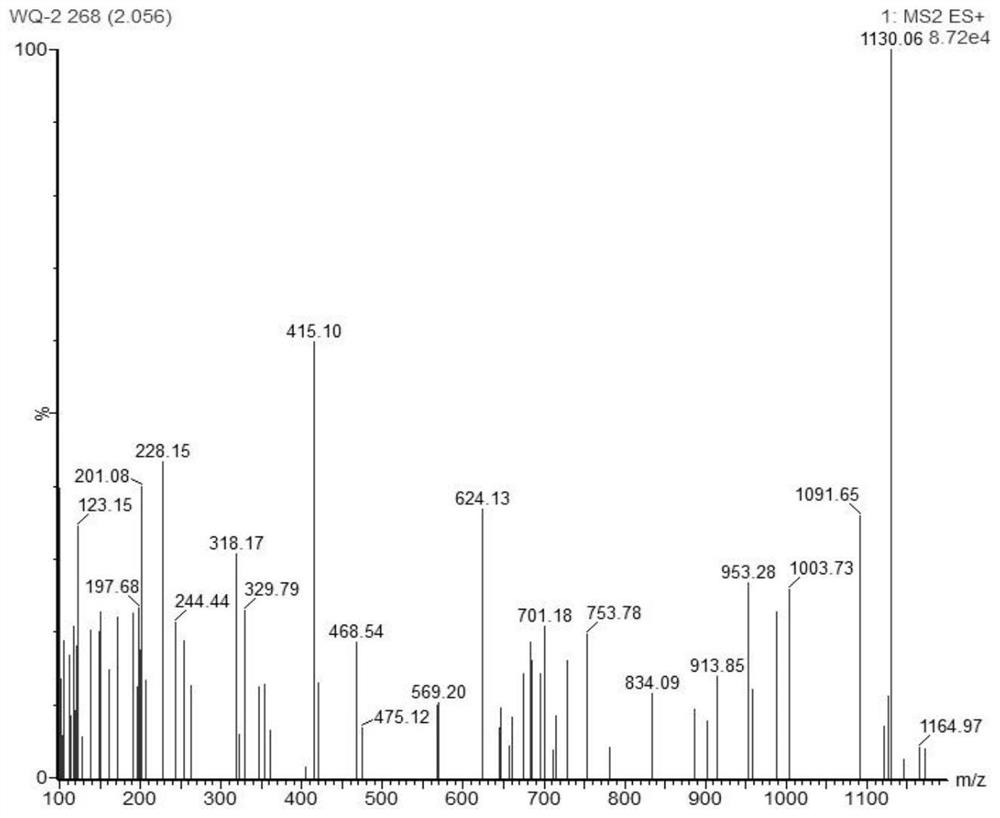
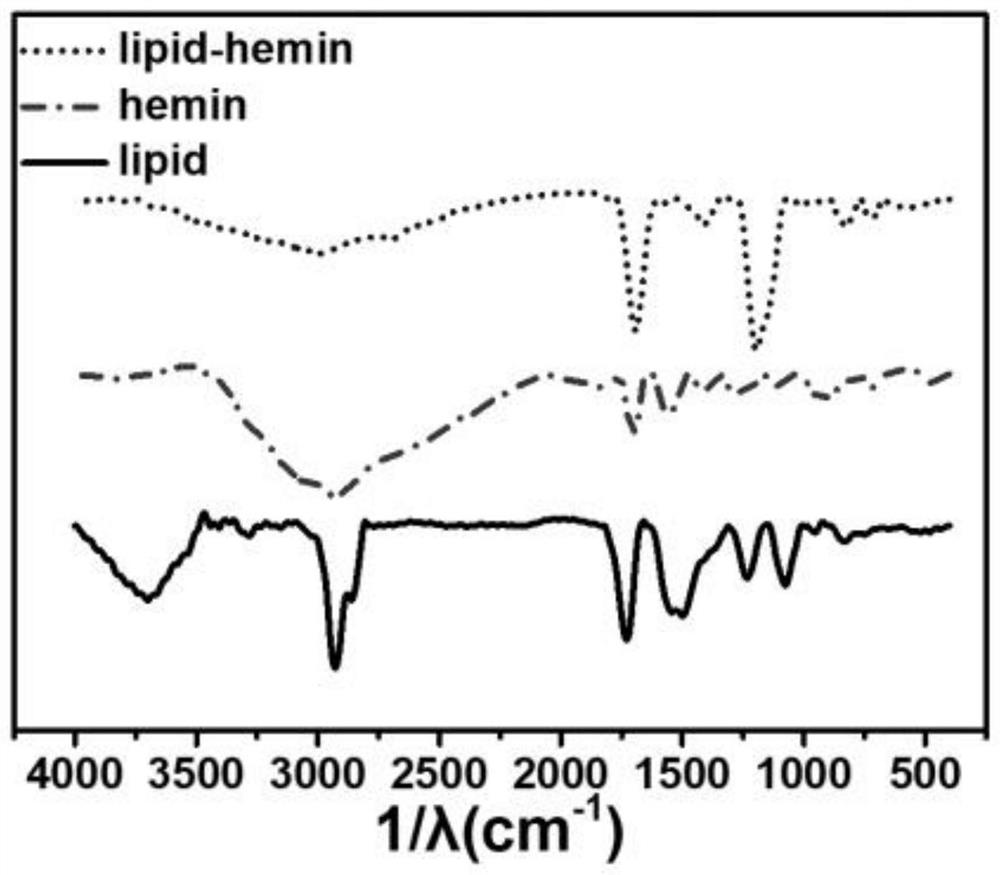
[0064] HPLC-MS characterization was performed on purified lipid-hemin, figure 1 As shown in, 1130.06 is LiPid-Hemin + H + , Indicating successful synthesis of lipid-hemin. In the infrared spectrum, through LiPID free hydroxyl restriction (wide peak, 3500 ~ 4000cm -1 ), ...
Example Embodiment
[0065] Example 2: Preparation method and characterization of FA-HEMESME-ART
[0066] 1, method:
[0067] According to the molar ratio of lipid-hemin-peg2000-fa = 85:13: 2, dissolved in chloroform: mixed solvents of methanol = 2: 1 (v: V); 10 mole% ART added In the above, in the above-mentioned phospholipid mixed liquid, the phospholip film is evaporated to give a phospholip film; 5 mL of phosphate buffer salt solution (PBS, pH 7.4), hydration of 1 h, freeze-thaw cycle; over 0.1 μm polycarbonate film The extruder is extruded, a 0.22 μm filter, ultrafiltration centrifugation 3000 rpm, 20 minutes, and purified FA-HeMesome-Art NPS.
[0068] 2, result
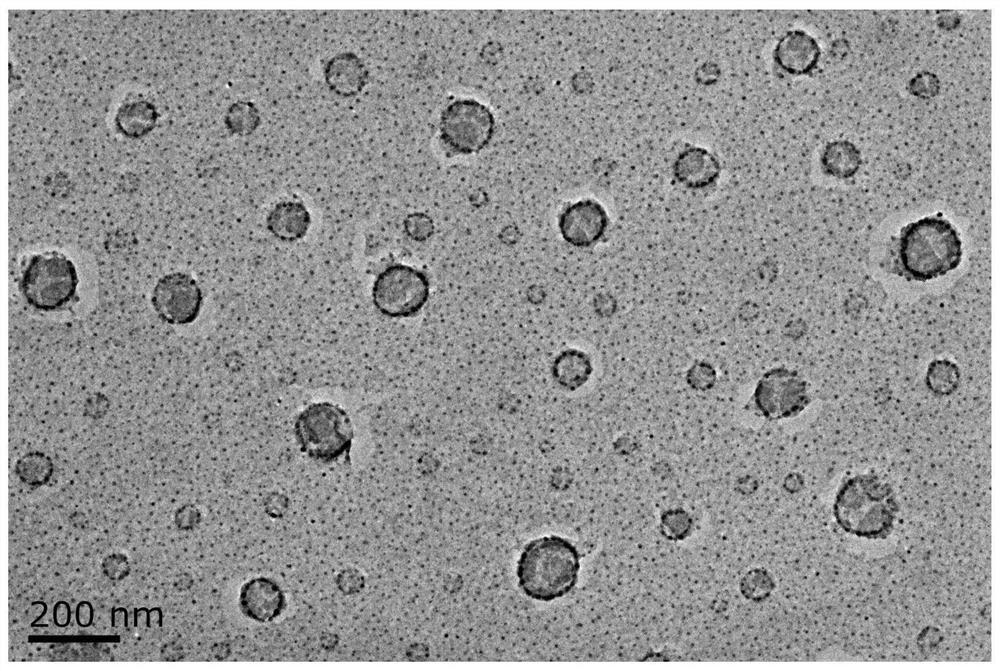
[0069] Its TEM morphology observation image 3 As shown, there is a spherical structure in size.
Example Embodiment
[0070] Example 3: Fa-Hemesome-ART nanoholic stability
[0071] 1, method
[0072] Under the conditions of PBS, SGF, SIF and medium (DMEM, 10% serum), in the process of incubation 48h, measurement of the hydrated particle size of FA-Hemesome-ART nanocolis (LiteSizer) during the corresponding time point (LiteSizer TM 500, Anton Paar, Austria.
[0073] 2, result
[0074] Under different environmental conditions, the particle size of FA-HEMESOME-ART NPS increases with incubation time as Figure 4 Indicated. Under the conditions described above, there was no significant change in particle size, which proved the stability of the nanocarburities in the gastrointestinal tract and blood. In addition, the size is slightly large in SIF and SGF, possibly due to increased particle size of the protein in the nanocarburization and the medium, but in an acceptable range, the intake of nanohububs and the in vivo absorption have little effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com