A VEGF antibody, reorganization AAV virus and its application
A technology of viruses and antibodies, applied in the field of biomedicine, can solve problems such as rarely used antibody structures, and achieve high proliferation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: VEGF antibody sequence design
[0035] according to figure 1 Design the VEGF antibody structure, connect the heavy chain and light chain of the antibody through the P2A linker, the heavy chain is at the 5' end of the gene sequence, and the light chain is at the 3' end of the gene.
[0036] The P2A linker is composed of the furin restriction site RKRR (arginine-lysine-arginine-arginine) and the P2A protein; after furin digestion, the residual amino acids of P2A remaining on the heavy chain are removed.
[0037] The P2A protein includes GSG (glycine-serine-glycine), which is located at the front of the P2A protein, and GSG (glycine-serine-glycine) is added to the P2A to improve the enzyme cutting efficiency of the P2A.
[0038] The heavy chain and light chain are composed of variable region and constant region, respectively, and the signal peptide is the signal peptide of human serum albumin, which is located at the 5' end of the heavy chain and light chain...
Embodiment 2
[0046] Embodiment 2: Construction of recombinant expression vector
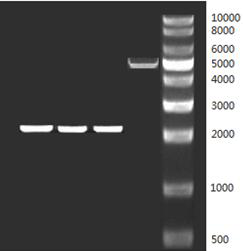
[0047] The sequences of mab (VEGF), ranibizumab, and G6 Fab that were commissioned to be synthesized were digested with NotI and HindIII respectively to obtain the target fragments with cohesive ends. At the same time, the vector pAAV-pCAG-wpre (purchased from addgene) was digested with NotI and HindIII to obtain a linearized vector fragment (see figure 2 ), use T4 ligase to connect the target fragment and the linearized vector fragment, transform it into E.coli (Top10), after the sequence is correct, use the plasmid extraction kit of OMEGA company to extract the plasmid, and obtain the recombinant expression vector pAAV-mab (VEGF) , pAAV-Raybead, pAAV-G6 Fab. The concentration of the recombinant expression vector pAAV-mab (VEGF) extracted in the present invention is 350ng / ul, the concentration of pAAV-Raybead is 325ng / ul, and the concentration of pAAV-G6 Fab is 315ng / ul.
Embodiment 3
[0048] Example 3: Culture and passage of packaging cell lines
[0049] The packaging cell line used in the present invention is 293T cells. Take out the frozen 293T cells from the liquid nitrogen tank, quickly throw them into a 37°C water bath and shake them quickly, try to dissolve the cell solution completely within 1-2 min. Transfer the cell solution to a 50ml centrifuge tube, add physiological saline to make the total volume 50ml, mix well and centrifuge at 1500 rpm for 5 min. Remove the supernatant, add 5 ml of fresh high-glucose DMEM (containing 10% FBS) medium to resuspend the cells, transfer them to T75 flasks, and fill each bottle with 10 ml of high-glucose DMEM (containing 10% FBS) medium. Place the culture flask steadily at 37°C, 5% CO 2 cultured in an incubator. The cell viability was observed the next day, and the medium was replaced. Afterwards, the growth of the cells was observed every day, and the cells were passaged when the cells covered 80%-90% of the b...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap