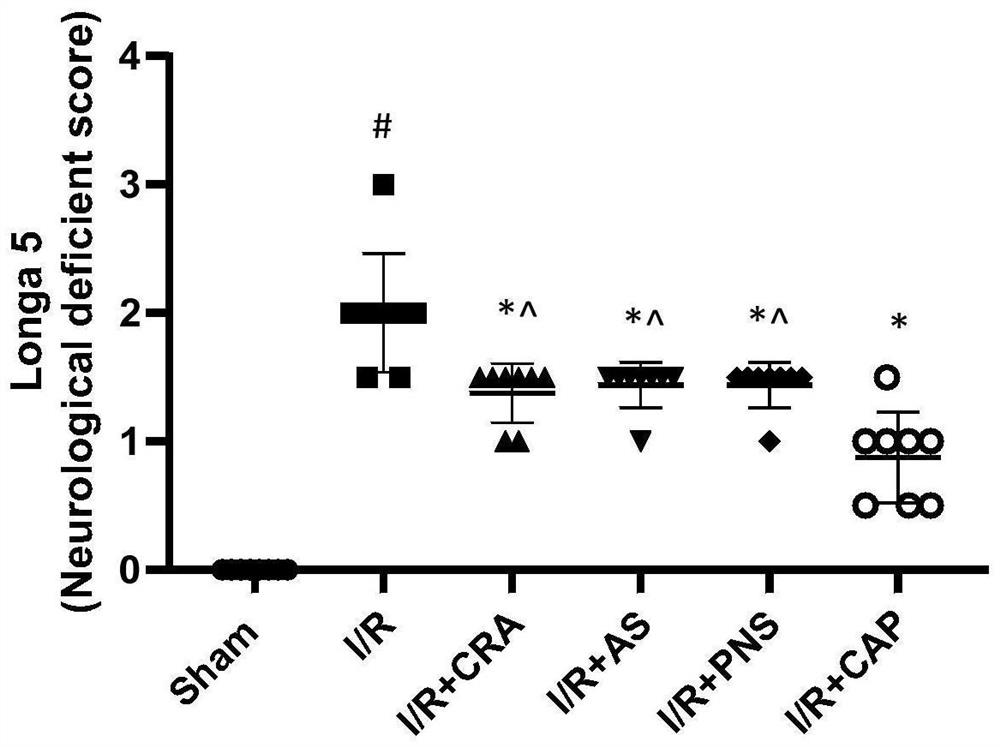
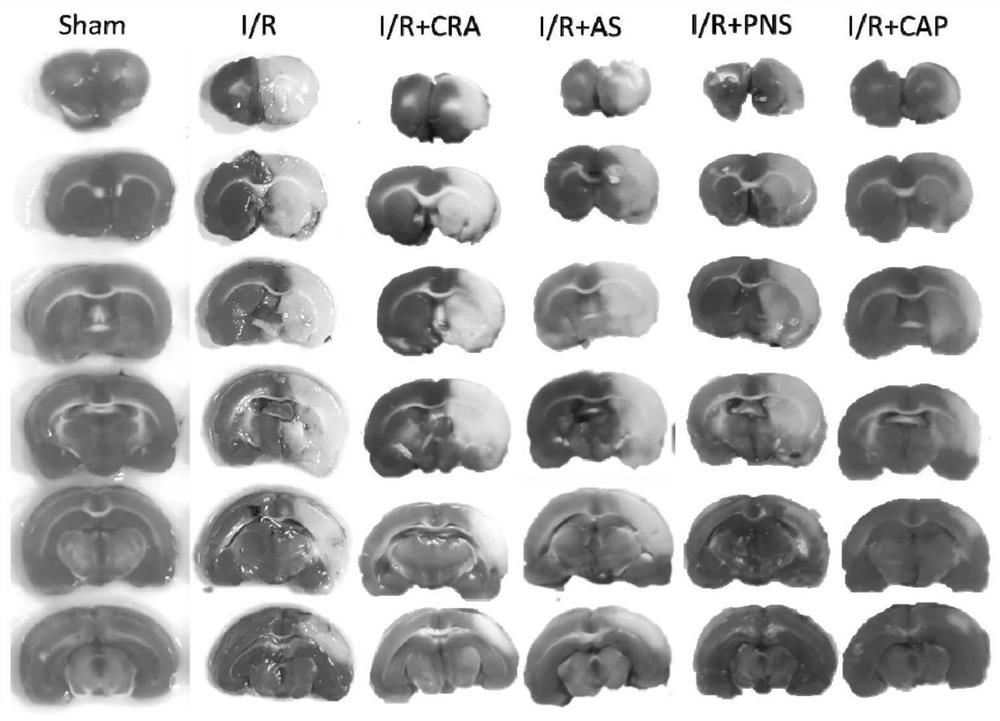
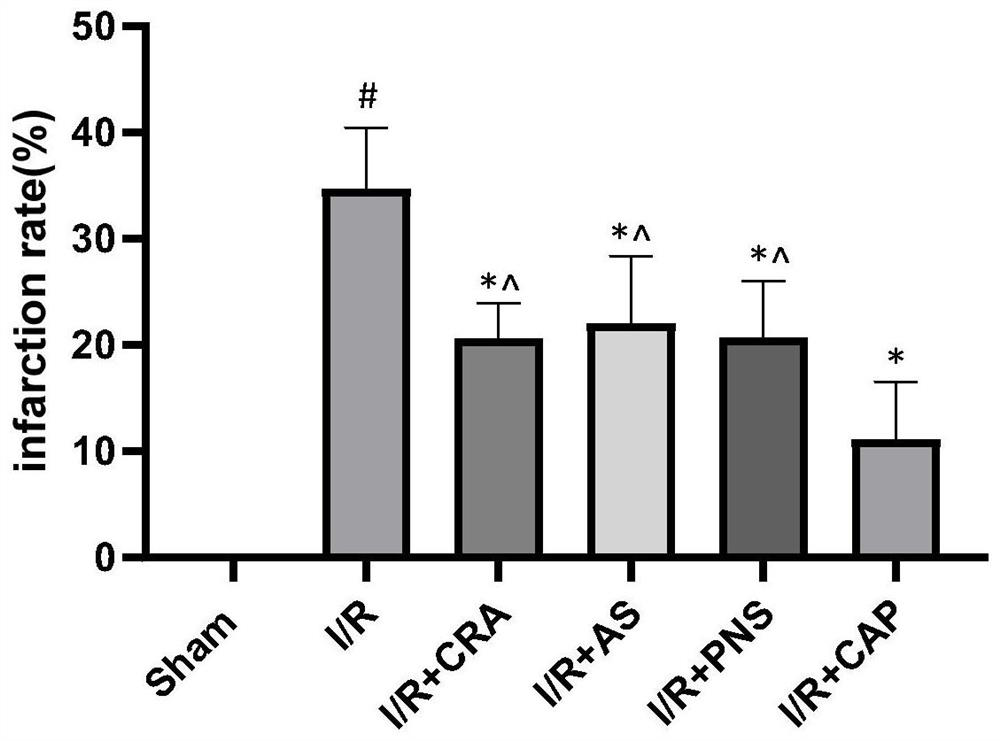
A kind of compound pharmaceutical composition and its application
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, plant raw materials, etc., can solve the problems that the therapeutic effect of the composition needs to be further improved, and the comprehensive curative effect needs to be improved, so as to achieve the effect of protecting from damage and improving the protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A compound pharmaceutical composition, in parts by weight, comprises the following components: 5 parts of Coptis chinensis total alkaloids, 5 parts of Astragalus total saponins and 1 part of Panax notoginseng saponins.
[0045] The preparation method of Coptis Coptidis total alkaloids comprises the following steps: extracting Coptis chinensis coarse powder with 80% (v / v) ethanol of 1 times the amount (solid-to-liquid ratio is 1 g / mL) at 25° C. for 24 hours, and extracting 3 times by the same method. Next, collect the supernatant after extraction, evaporate to dryness under a rotary evaporator, and obtain Coptis chinensis total alkali.
Embodiment 2
[0047] A compound pharmaceutical composition, in parts by weight, comprises the following components: 10 parts of Coptis chinensis total alkaloids, 10 parts of Astragalus total saponins and 3 parts of Panax notoginseng total saponins.
[0048] The preparation method of Coptis Coptidis total alkaloids comprises the following steps: extracting Coptis Coptidis coarse powder with 75% (v / v) ethanol of 1.2 times the amount (solid-liquid ratio is 1:1.2 g / mL) at 30° C. for 30 hours, and using the same Method Extract 4 times, collect the supernatant after extraction, evaporate to dryness under a rotary evaporator, and obtain Coptis chinensis total alkaloids.
Embodiment 3
[0050] A compound pharmaceutical composition, calculated in parts by weight, comprises the following components: 8 parts of Coptis chinensis total alkaloids, 8 parts of astragalus total saponins and 2 parts of Panax notoginseng saponins.
[0051] The preparation method of Coptis Coptidis total alkaloids comprises the following steps: extracting Coptis Coptidis coarse powder with 85% (v / v) ethanol of 0.8 times the amount (solid-liquid ratio is 1:0.8 g / mL) at 20° C. for 20 hours, and using the same Method Extract twice, collect the supernatant after extraction, evaporate to dryness under a rotary evaporator, and obtain Coptis chinensis total alkaloids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com