Ramelteon sustained-release preparation and preparation method thereof
A technology for ramelteon and sustained-release preparations, applied in the field of ramelteon sustained-release preparations and its preparation, can solve the problems of affecting the patient's mental state, short drug effect maintenance time, and reducing sleep quality, so as to achieve sufficient sleep time , avoid premature awakening, simple craft effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The present invention also provides a preparation method of the above-mentioned ramelteon sustained-release preparation, comprising the steps of:
[0068] Mix ramelteon, filler and extended release material, then add lubricant and mix.
[0069] In a specific example, the following step is further included after mixing: performing wet granulation with ethanol or water.
[0070] In a specific example, the blending is followed by compression into a tablet.
[0071] In a specific example, capsules are filled after mixing.
[0072] In a specific example, granules are prepared after mixing and filling sachets.
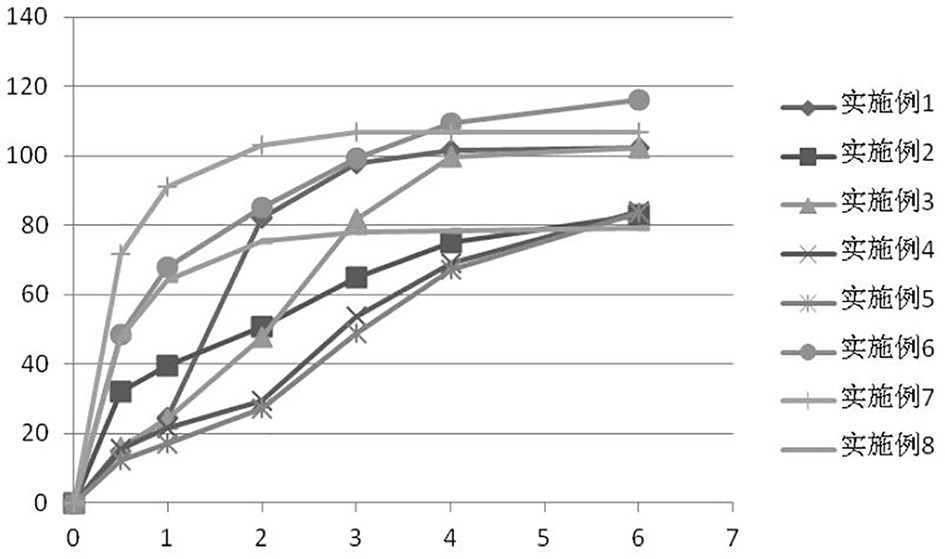
Embodiment 1
[0075] The present embodiment provides a sustained-release preparation of ramelteon, and the specific prescription is as follows:
[0076]
[0077] Concrete preparation method is as follows:
[0078] Mix ramelteon, lactose, microcrystalline cellulose PH101, hyprolose Klucel EXF PHARM, and silicon dioxide evenly. Slowly add 95% ethanol solution to the above mixture, stirring while adding. The soft material is suitable for softness and hardness, and it forms a ball when you hold it, and it disperses when you touch it lightly. The soft material was granulated through a 10-mesh sieve, and the prepared granules were dried in an oven at 60°C. After drying for 1 hour, measure the loss on drying of the granules, and stop drying when the loss on drying is less than 3%. The dried granules are then added with magnesium stearate and mixed evenly. Compressed tablet, tablet weight 250mg, hardness 3~10kg.
Embodiment 2
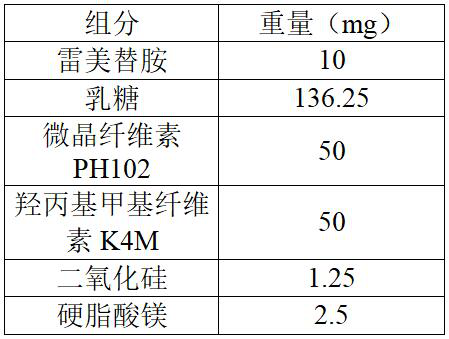
[0080] The present embodiment provides a sustained-release preparation of ramelteon, and the specific prescription is as follows:
[0081]
[0082] Concrete preparation method is as follows:
[0083] Mix ramelteon, lactose, microcrystalline cellulose PH102, hydroxypropyl methylcellulose K4M, and silicon dioxide evenly. Then add magnesium stearate and mix well. Compressed tablet, tablet weight 250mg, hardness 3~10kg.
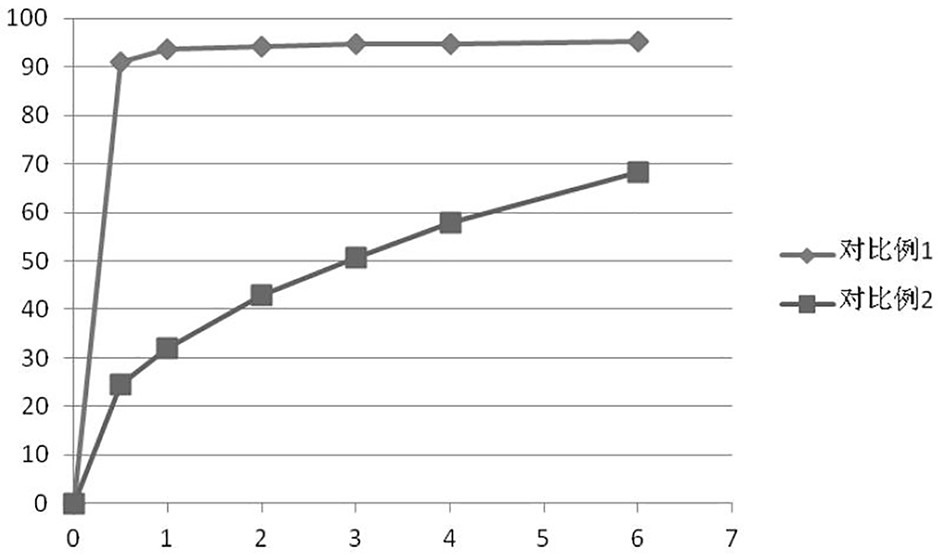
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com