Prescription of tranilast emulsifiable paste and preparation process of tranilast emulsifiable paste
A special cream and tranis technology, applied in the field of medicine, can solve the problems of insufficient blood drug concentration in local scar tissue, unfavorable patient compliance, and slow onset of effect, so as to reduce the risk of systemic adverse reactions and improve patient compliance Sexuality, the effect of avoiding liver and kidney toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
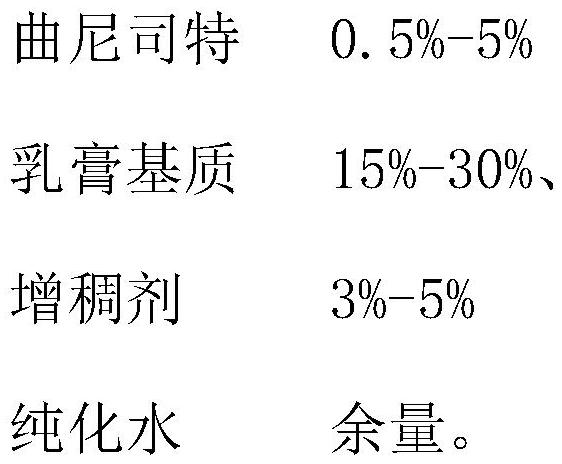
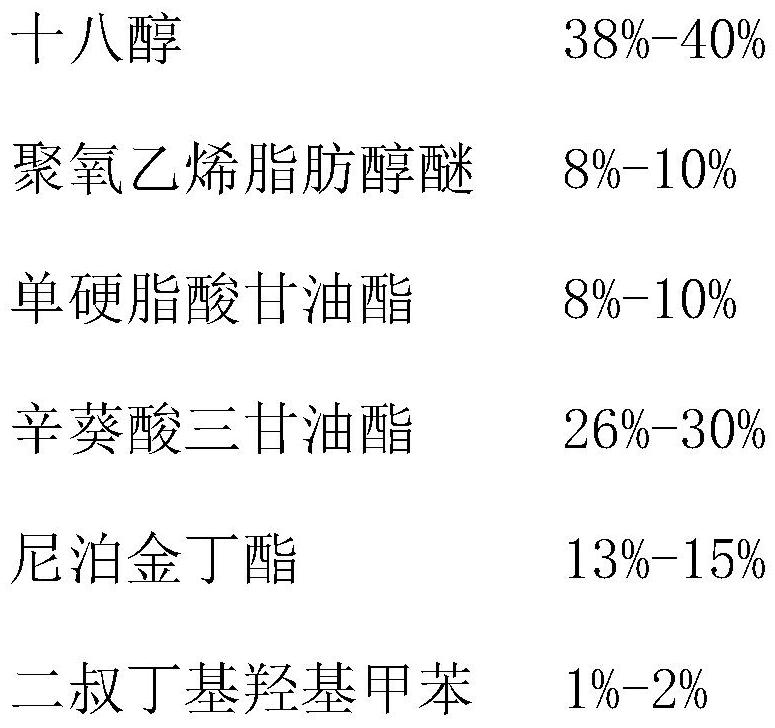
[0027] (1) Preparation of oil phase liquid: stearyl alcohol 90g, polyoxyethylene fatty alcohol ether 20g, glyceryl monostearate 20g, caprylic triglyceride 65g, butylparaben 30g, di-tert-butyl The ratio of 5g of hydroxytoluene is added to the oil phase pot in turn, and when the temperature rises to 85-90°C, turn off the heating and wait for use;
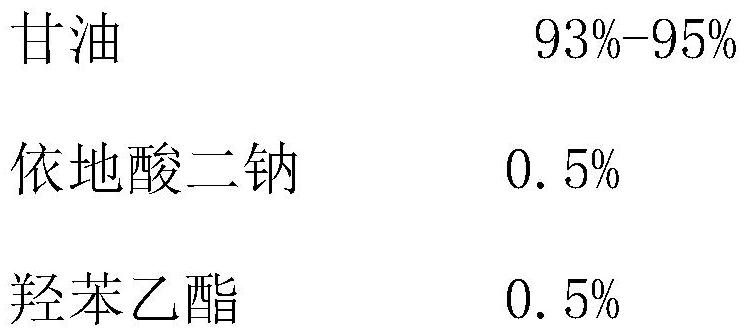
[0028] (2) Preparation of water phase liquid: Add 200g of glycerin, 500g of purified water, 1g of edetate disodium, and 1g of ethylparaben to the water phase pot in sequence. When the temperature of the water phase reaches 90-95°C, add Sodium lauryl sulfate 10g, stir until the material dissolves, turn off the stirring;
[0029] (3) Emulsification: first transfer all the oil phase substances to the vacuum emulsification pot, and then slowly transfer all the water phase substances to the emulsification pot. The cooling water starts to cool;
[0030] (4) Preparation of tranilast dispersion: disperse 10 g of tranilast and 1 g of sodium ...
Embodiment 2
[0034] (1) Preparation of oil phase liquid: stearyl alcohol 90g, polyoxyethylene fatty alcohol ether 20g, glyceryl monostearate 20g, caprylic triglyceride 65g, butylparaben 30g, di-tert-butyl The ratio of 5g of hydroxytoluene is added to the oil phase pot in turn, and when the temperature rises to 85-90°C, turn off the heating and wait for use;
[0035] (2) Preparation of water phase liquid: Add 200g of glycerin, 500g of purified water, 1g of edetate disodium, and 1g of ethylparaben to the water phase pot in sequence. When the temperature of the water phase reaches 90-95°C, add Sodium lauryl sulfate 10g, stir until the material dissolves, turn off the stirring;
[0036] (3) Emulsification: first transfer all the oil phase substances to the vacuum emulsification pot, and then slowly transfer all the water phase substances to the emulsification pot. The cooling water starts to cool;
[0037] (4) Preparation of tranilast dispersion: disperse 20 g of tranilast and 1 g of sodium ...
Embodiment 3
[0041] Production of Hypertrophic Scar Model in Rabbit Ears
[0042] After the animal was adaptively fed for 1 week, the production of the scar model referred to the literature method (Bian Huining, Li Tianzeng, Xie Julin, etc. Effect of basic fibroblast growth factor on the formation of rabbit ear hypertrophic scar. Chinese Journal of Trauma, 2004, 20(4):242-245) and improved, with 30g·L -1 Sodium pentobarbital 1.0mL·kg -1 (30mg·kg -1 ) for auricular vein anesthesia, iodophor and ethanol disinfection of the ventral skin of the rabbit ear, avoiding visible blood vessels along the long axis of the ventral middle section of the rabbit ear, and gently drilling a 1cm×1cm wound with a corneal ring, with an interval of more than 1cm between the wounds, The full-thickness skin of the rabbit ears was removed and the perichondrium was completely scraped off with a spatula. Sterile cotton balls were pressed to stop the bleeding, 4 places per ear. The postoperative wounds were exposed....
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap