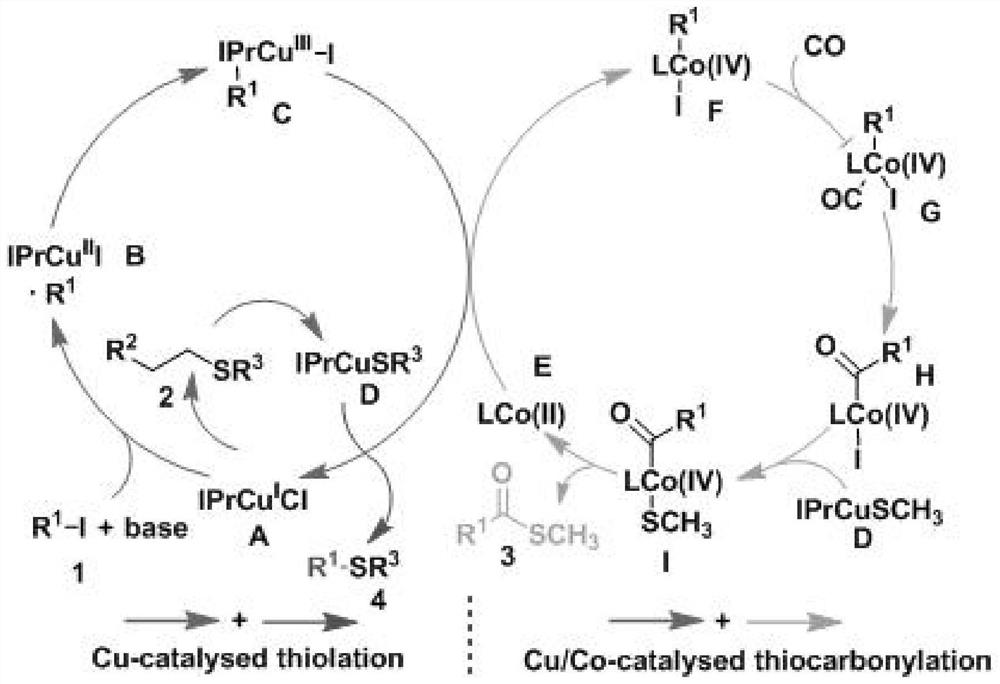
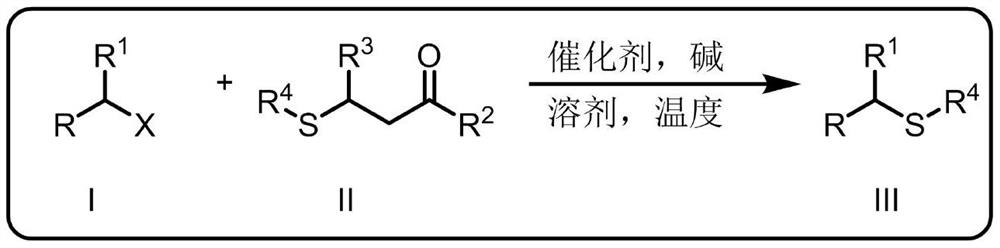
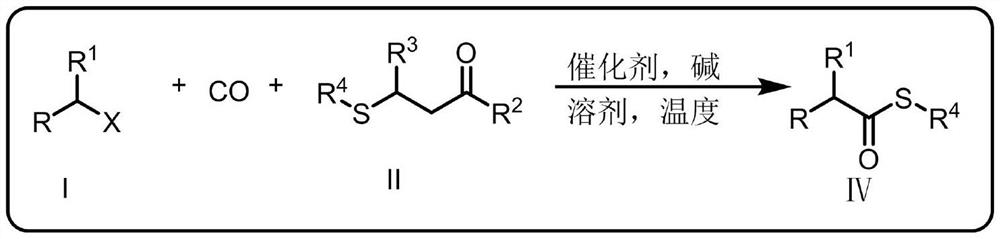
A kind of synthetic method of thioetherification and thiocarbonylation of halogenated alkanes
A technology of halogenated alkane sulfide and synthesis method, which is applied in the synthesis field of halogenated alkane thioetherification and thiocarbonylation, can solve the problems of complicated preparation of substrates, low yield, unpleasant odor and high toxicity of mercaptan, and the like, The method is simple, economical and practical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] To prepare 2-methylthio-4-p-methylphenylbutane, the reaction scheme is as follows:
[0042]
[0043] Add IPrCuCl (4.87mg, 0.01mol), NaO t Bu (76.8mg, 4equiv.) and dried stir bar. charging N 21-(3-Iodobutyl)-4-methylbenzene (0.2 mmol, 1 equiv.), methyl 3-(methylthio)propionate (0.6 mmol, 3 equiv.) and xylene (2 mL) were added under the same conditions. The resulting solution was heated to 140°C and the reaction was stirred at this temperature for 24 hours. Then, cool to room temperature. The solvent was removed under reduced pressure, and the crude product was purified by silica gel column chromatography, eluting with petroleum ether, and a colorless liquid (31 mg, 80%) was isolated.
[0044] The proton nuclear magnetic resonance spectrum analysis result is as follows:
[0045] 1 H NMR (600MHz, Chloroform-d) δ7.10(d, J=4.9Hz, 4H), 2.75–2.69(m, 2H), 2.66(p, J=6.8Hz, 1H), 2.33(d, J= 4.8Hz, 3H), 2.07(d, J=4.9Hz, 3H), 1.87(m, 1H), 1.77(m, 1H), 1.31(d, J=6.7Hz, 3H)....
Embodiment 2
[0053] To prepare 4-methylthiophenvalyl ether, the reaction scheme is as follows:
[0054]
[0055] Add IPrCuCl (4.87mg, 0.01mmol), NaO t Bu (76.8mg, 4equiv.) and dried stir bar. charging N 2 (4-Iodopentyloxy)benzene (0.2 mmol, 1 equiv.), methyl 3-(methylthio)propionate (0.6 mmol, 3 equiv.) and xylene (2 mL) were added under the same conditions. The resulting solution was heated to 140°C and the reaction was stirred at this temperature for 24 hours. Then, cool to room temperature. The solvent was removed under reduced pressure, and the crude product was purified by silica gel column chromatography, eluting with petroleum ether, and a colorless liquid (35 mg, 83%) was isolated.
[0056] The proton nuclear magnetic resonance spectrum analysis result is as follows:
[0057] 1 H NMR (600MHz, Chloroform-d) δ7.27(m,2H),7.01–6.80(m,3H),3.97(m,2H),2.73(h,J=6.8Hz,1H),2.08(s, 3H), 1.96–1.84 (m, 2H), 1.78–1.62 (m, 2H), 1.31 (d, J=6.7Hz, 3H).
[0058] The carbon NMR analysis re...
Embodiment 3
[0065] To prepare 2-methylthio-4-ethoxyphenylbutane, the reaction scheme is as follows:
[0066]
[0067] Add IPrCuCl (4.87mg, 0.01mmol), NaO t Bu (76.8mg, 4equiv.) and dried stir bar. charging N 2 Add 1-ethoxy-4-(3-iodobutyl)benzene (0.2mmol, 1equiv.), methyl 3-(methylthio)propanoate (0.6mmol, 3equiv.) and xylene (2mL) . Methyl 3-(methylthio)propionate (0.6 mmol, 3 equiv.) and xylene (2 mL). The resulting solution was heated to 140°C and the reaction was stirred at this temperature for 24 hours. Then, cool to room temperature. The solvent was removed under reduced pressure, and the crude product was purified by silica gel column chromatography, eluting with petroleum ether, and a colorless liquid (33 mg, 74%) was isolated.
[0068] The proton nuclear magnetic resonance spectrum analysis result is as follows:
[0069] 1 H NMR (600MHz, Chloroform-d) δ7.15–7.05(m,2H),6.85–6.76(m,2H),4.01(q,J=7.0Hz,2H),2.77–2.55(m,3H), 2.06(s, 3H), 1.85(m, 1H), 1.74(m, 1H), 1.39(t, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com