A kind of preparation method of cyanated or thiocyanated indolizine and derivatives thereof
A derivative, the technology of pyrazine, which is applied in the field of organic synthesis, can solve the problems of large doses of oxidants and heavy metal waste, and achieve the effects of wide application range, simple operation and good regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
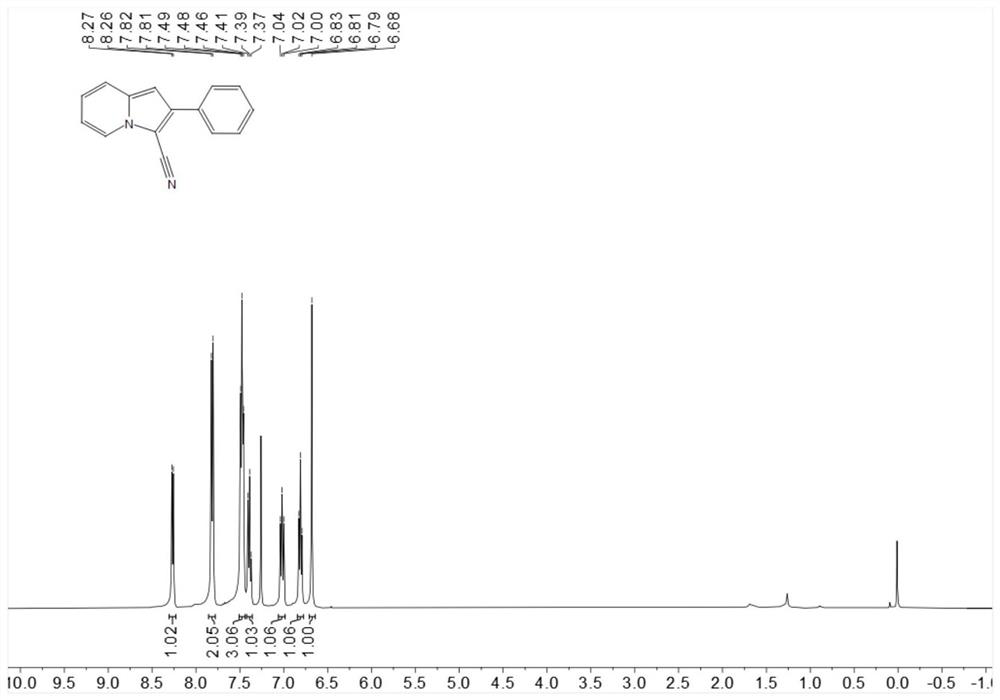
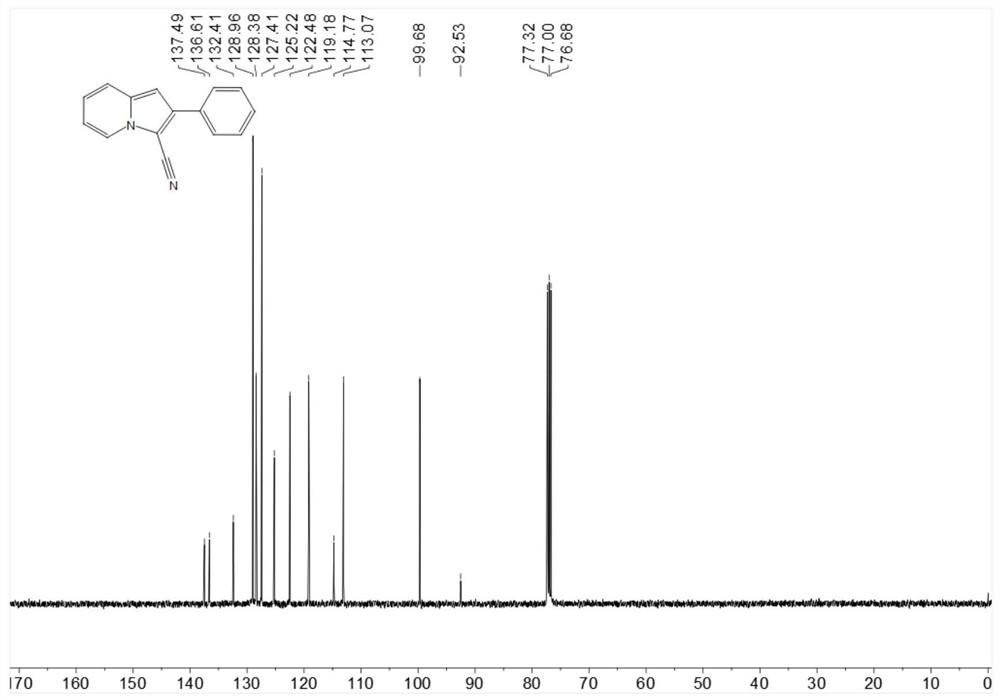
[0218] Place 19.3mg (0.1mmol) 2-phenylindolizine, 1.1mg-10.2mg (0.001mmol-0.01mmol) Rose Bengal, 4.6mg-22.8mg (0.06mmol-0.3mmol) ammonium thiocyanate in 25ml In a test tube with a stirring bar, add 1 mL of 1,2-dichloroethane at room temperature, then add 3.4 mg-5.1 mg (0.1 mmol-0.15 mmol) of hydrogen peroxide, stir under blue light for 6 hours, spin dry The target product was obtained by post-flash column chromatography in 76% yield.
[0219]
[0220] 1H NMR (400MHz, Chloroform-d) δ8.27(d, J=6.9Hz, 1H), 7.81(d, J=7.7 Hz, 2H), 7.51-7.44(m, 3H), 7.39(t, J= 7.4Hz, 1H), 7.06–6.98 (m, 1H), 6.81 (t, J = 6.8Hz, 1H), 6.68 (s, 1H).
[0221] 13C NMR (101MHz, Chloroform-d) δ137.5, 136.6, 132.4, 128.96, 128.4, 127.4, 125.2, 122.5, 119.2, 114.8, 113.1, 99.7, 92.5.
[0222] HR-ESI-MS m / z calcd.for C15H10N2[M+H]+:219.0922,found:219.0922.
Embodiment 2
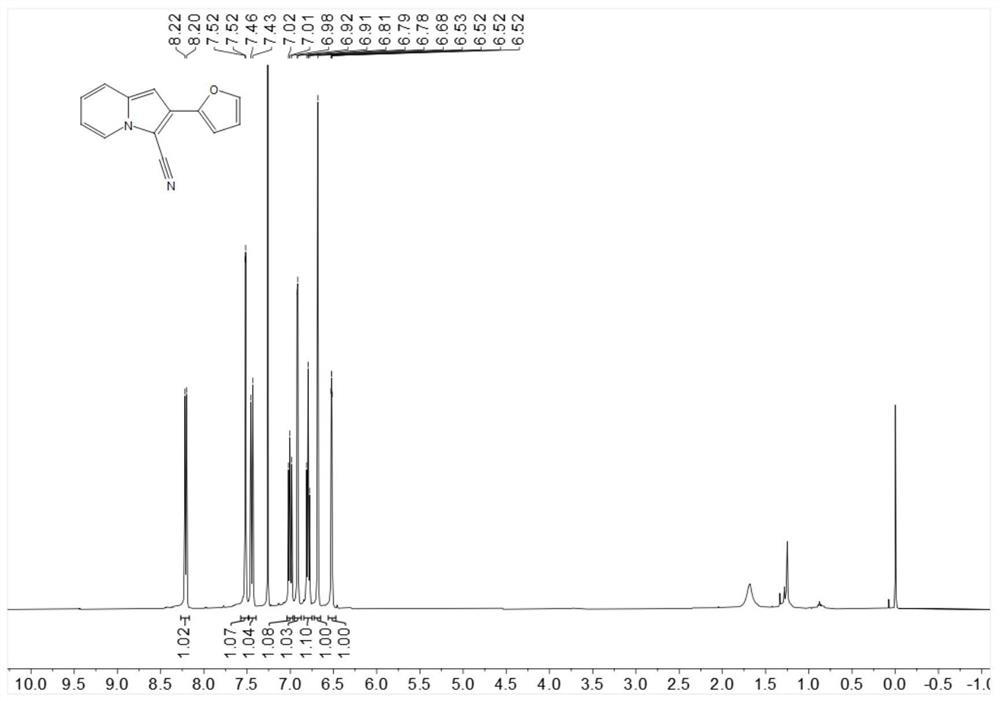
[0224] Put 18.3mg (0.1mmol) 2-furyl indolizine, 1.1mg-10.2mg (0.001mmol-0.01mmol) Rose Bengal, 4.6mg-22.8mg (0.06mmol-0.3mmol) ammonium thiocyanate in 25ml In a test tube with a stirring bar, add 1 mL of 1,2-dichloroethane at room temperature, then add 3.4 mg-5.1 mg (0.1 mmol-0.15 mmol) of hydrogen peroxide, stir under blue light for 6 hours, spin dry The target product was obtained by post-flash column chromatography in 64% yield.
[0225]
[0226] 1H NMR (400MHz, Chloroform-d) δ8.21 (d, J=6.9Hz, 1H), 7.52 (d, J=1.5 Hz, 1H), 7.45 (d, J=9.0Hz, 1H), 7.04–6.97 (m, 1H), 6.92 (d, J=3.4Hz, 1H), 6.79 (t, J=6.8Hz, 1H), 6.68 (s, 1H), 6.52 (dd, J=3.3, 1.8Hz, 1H) .
[0227]13C NMR (101MHz, Chloroform-d) δ147.3, 142.6, 136.6, 127.8, 125.2, 122.6, 119.2, 114.2, 113.2, 111.8, 108.1, 97.5, 91.0.
[0228] HR-ESI-MS m / z calcd.for C13H8N2O[M+H]+:209.0719,found:209.0715.
Embodiment 3
[0230] 27.1mg (0.1mmol) 2-(4-bromophenyl) indolizine, 1.1mg-10.2mg (0.001mmol-0.01mmol) Rose Bengal, 4.6mg-22.8mg (0.06mmol-0.3mmol) thiocyanate Ammonium acid was placed in a 25ml test tube with a stirrer, and 1mL of 1,2-dichloroethane was added at room temperature, followed by 3.4mg-5.1mg (0.1mmol-0.15mmol) hydrogen peroxide, and stirred under blue light irradiation. After 6 hours, the target product was obtained by flash column chromatography after spin-drying in 63% yield.
[0231]
[0232] 1H NMR(400MHz, Chloroform-d)δ8.28(d,J=6.9Hz,1H),7.68(d,J=8.2Hz,2H),7.60(d,J=8.4Hz,2H),7.50(d , J=8.9Hz, 1H), 7.07–7.01(m, 1H), 6.85 (t, J=6.8Hz, 1H), 6.66(s, 1H).
[0233] 13C NMR (101MHz, Chloroform-d) δ136.7, 136.3, 132.2, 131.5, 129.0, 125.4, 122.8, 122.6, 119.3, 113.4, 99.7.
[0234] HR-ESI-MS m / z calcd.for C15H9BrN2[M+H]+:297.0029,found:297.0027.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com