Preparation method of cyanated or thiocyanated indolizine and derivatives thereof
A derivative, cyanation technology, applied in chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, chemical/physical processes, etc. To achieve the effect of simple operation, wide application range and good regional selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
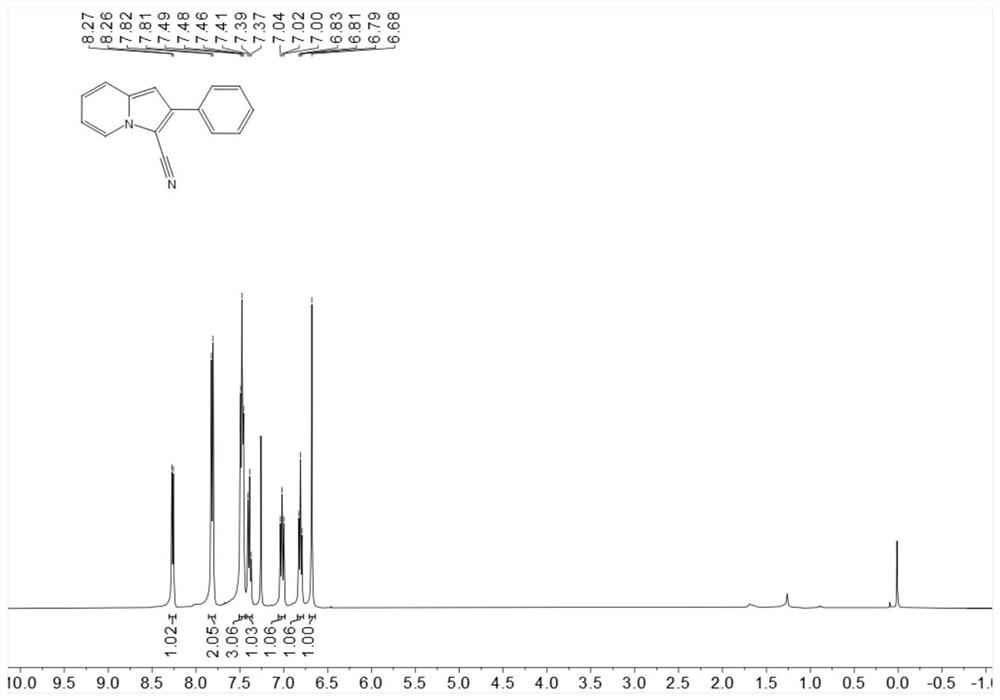
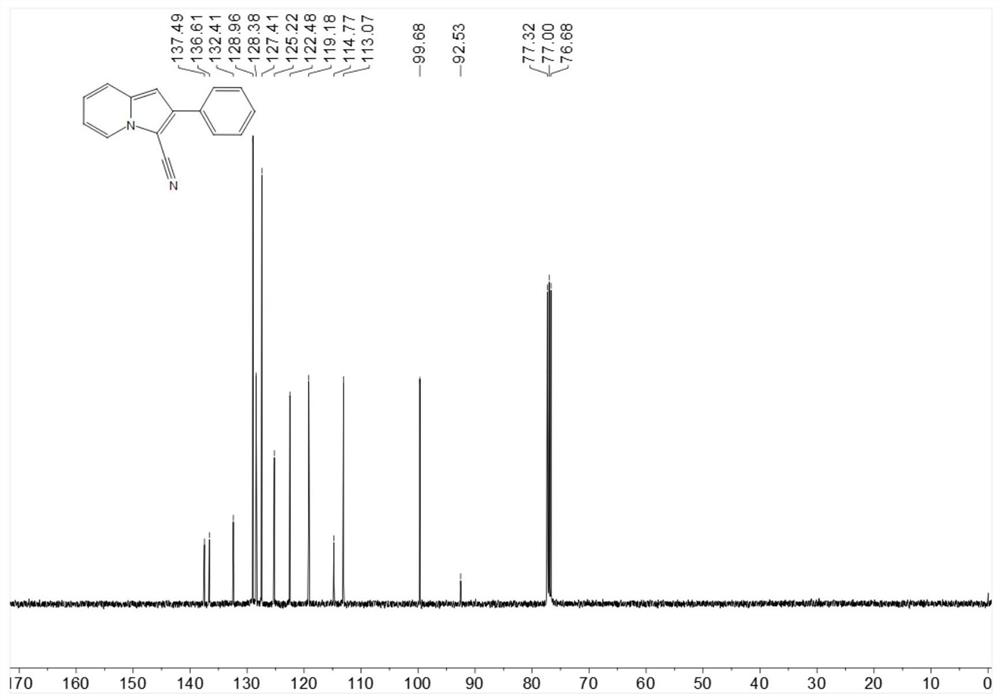
[0218] Put 19.3mg (0.1mmol) 2-phenylindolizine, 1.1mg-10.2mg (0.001mmol-0.01mmol) Bengal rose, 4.6mg-22.8mg (0.06mmol-0.3mmol) ammonium thiocyanate in 25ml In a test tube with a stirring bar, add 1mL of 1,2-dichloroethane at room temperature, then add 3.4mg-5.1mg (0.1mmol-0.15mmol) of hydrogen peroxide, stir for 6 hours under blue light irradiation, spin dry After flash column chromatography, the target product was obtained in 76% yield.
[0219]
[0220] 1H NMR (400MHz, Chloroform-d) δ8.27(d, J=6.9Hz, 1H), 7.81(d, J=7.7 Hz, 2H), 7.51–7.44(m, 3H), 7.39(t, J= 7.4Hz, 1H), 7.06–6.98(m, 1H), 6.81(t, J = 6.8Hz, 1H), 6.68(s, 1H).
[0221] 13C NMR (101MHz, Chloroform-d) δ137.5, 136.6, 132.4, 128.96, 128.4, 127.4, 125.2, 122.5, 119.2, 114.8, 113.1, 99.7, 92.5.
[0222] HR-ESI-MS m / z calcd.for C15H10N2[M+H]+:219.0922,found:219.0922.
Embodiment 2
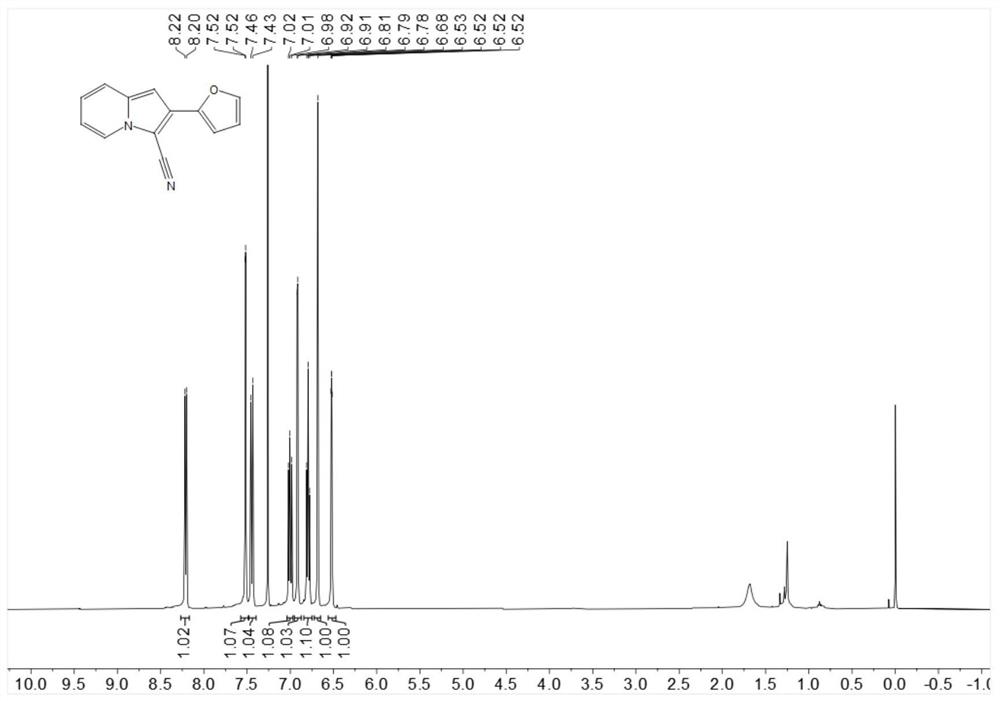
[0224] Put 18.3mg (0.1mmol) 2-furyl indolizine, 1.1mg-10.2mg (0.001mmol-0.01mmol) Rose Bengal, 4.6mg-22.8mg (0.06mmol-0.3mmol) ammonium thiocyanate in 25ml In a test tube with a stirring bar, add 1mL of 1,2-dichloroethane at room temperature, then add 3.4mg-5.1mg (0.1mmol-0.15mmol) of hydrogen peroxide, stir for 6 hours under blue light irradiation, spin dry After flash column chromatography, the target product was obtained in 64% yield.
[0225]
[0226] 1H NMR (400MHz, Chloroform-d) δ8.21 (d, J = 6.9Hz, 1H), 7.52 (d, J = 1.5 Hz, 1H), 7.45 (d, J = 9.0Hz, 1H), 7.04–6.97 (m,1H),6.92(d,J=3.4Hz,1H),6.79 (t,J=6.8Hz,1H),6.68(s,1H),6.52(dd,J=3.3,1.8Hz,1H) .
[0227]13C NMR (101MHz, Chloroform-d) δ147.3, 142.6, 136.6, 127.8, 125.2, 122.6, 119.2, 114.2, 113.2, 111.8, 108.1, 97.5, 91.0.
[0228] HR-ESI-MS m / z calcd. for C13H8N2O[M+H]+: 209.0719, found: 209.0715.
Embodiment 3
[0230] 27.1mg (0.1mmol) 2-(4-bromophenyl) indolizine, 1.1mg-10.2mg (0.001mmol-0.01mmol) Bengal rose, 4.6mg-22.8mg (0.06mmol-0.3mmol) thiocyanate Ammonium chloride is placed in a 25ml test tube with a stirring bar, after adding 1mL of 1,2-dichloroethane at room temperature, then add 3.4mg-5.1mg (0.1mmol-0.15mmol) of hydrogen peroxide, and stir under blue light irradiation After 6 hours, the target product was obtained by flash column chromatography after spin-drying, with a yield of 63%.
[0231]
[0232] 1H NMR (400MHz, Chloroform-d) δ8.28(d, J=6.9Hz, 1H), 7.68(d, J=8.2 Hz, 2H), 7.60(d, J=8.4Hz, 2H), 7.50(d ,J=8.9Hz,1H),7.07–7.01(m,1H),6.85 (t,J=6.8Hz,1H),6.66(s,1H).
[0233] 13C NMR (101MHz, Chloroform-d) δ136.7, 136.3, 132.2, 131.5, 129.0, 125.4, 122.8, 122.6, 119.3, 113.4, 99.7.
[0234] HR-ESI-MS m / z calcd. for C15H9BrN2[M+H]+: 297.0029, found: 297.0027.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com