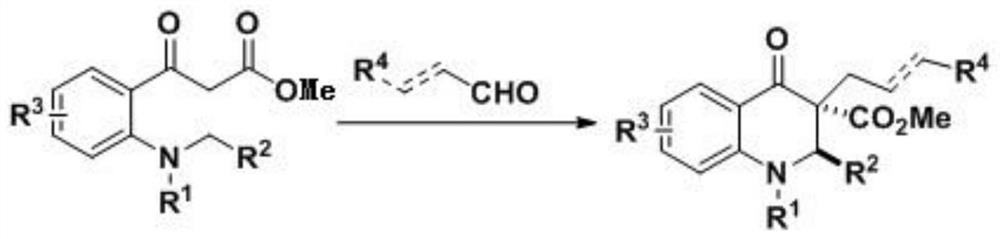
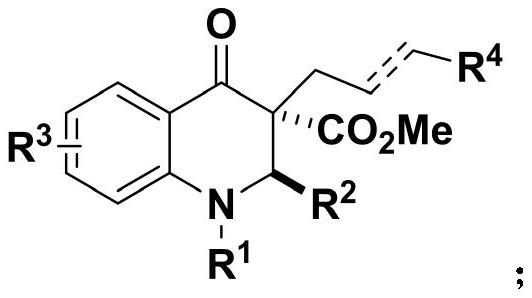
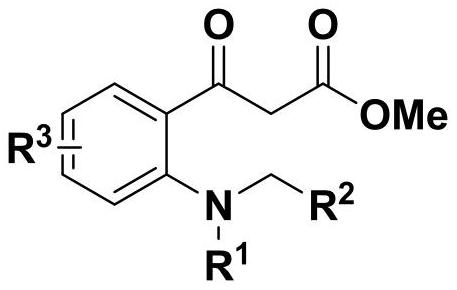
2, 3-dihydroquinoline-4-ketone bioactive skeleton as well as synthesis method and application thereof
A synthetic method and technology of dihydroquinoline, applied in drug combination, non-central analgesics, organic chemistry, etc., can solve the problems of long reaction time of metal catalysts, poor substrate universality, and high price of chiral phosphoric acid, etc. Achieve good practical significance and application value, good substrate universality, green and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. The present embodiment provides a method for synthesizing a 2,3-dihydroquinolin-4-one biologically active skeleton, comprising the following steps:
[0048]Take 0.1 mmol of methyl 2-aminobenzoylacetate compound in a reaction flask, and add 1 mL of solvent, 0.15 mmol of formaldehyde compound and catalyst in sequence. The reaction temperature of the system was controlled, stirring was continued, and the reaction was followed by spotting on a thin-layer chromatographic plate until the reaction of the raw materials was complete. After the reaction is completed, the silica gel column is used for separation and purification, and the purified product is rotary evaporated to obtain the target product. The reaction formula is as follows:
[0049]
[0050] 2. According to the above method, 9 groups of parallel test groups were set up, respectively using different catalysts and solvents. The catalysts are acetic acid / ammonium acetate Ac(OH) / NH respectively 4 OAc, Piperidi...
Embodiment 2
[0061] Raw materials: methyl 2-tetrahydropyrrole benzoylacetate, benzaldehyde
[0062] Product: chemical formula C 21 H 21 NO 3
[0063] Molecular weight: 335.4030
[0064] Structural formula:
[0065] Yield: 79%
[0066] 1 H NMR (500MHz, CDCl 3 )δ7.69(d,J=7.9Hz,1H),7.38(t,J=7.7Hz,1H),7.09–7.02(m,3H),6.94(d,J=4.3Hz,2H),6.67( dd,J=18.1,10.7Hz,1H),6.57(d,J=8.4Hz,1H),4.17(dd,J=9.5,5.6Hz,1H),3.81(s,3H),3.48(ddd,J =17.8,15.6,8.9Hz,2H),3.32(d,J=13.7Hz,1H),2.90(d,J=13.7Hz,1H),2.20–2.12(m,1H),2.09–1.91(m, 3H). 13 C NMR (126MHz, CDCl 3 )δ190.86(s), 171.96(s), 148.38(s), 136.30(s), 135.54(s), 130.71(s), 129.17(s), 127.47(s), 126.46(s), 118.29( s), 116.59(s), 112.73(s), 77.34(d, J=6.5Hz), 77.11(s), 76.86(s), 63.44(s), 61.74(s), 52.60(s), 46.83( s), 31.74(s), 26.23(s), 22.95(s).HRMS(ESI): calcd for C 21 H 21 NO 3 Na[M+Na] + :358.3922,found:358.3924.
Embodiment 3
[0068] Raw materials: methyl 2-tetrahydropyrrole benzoylacetate, p-nitrobenzaldehyde
[0069] Product: chemical formula C 21 H 20 N 2 O 5
[0070] Molecular weight: 380.1372
[0071] Structural formula:
[0072] Yield: 72%
[0073] 1 H NMR (500MHz, CDCl 3 )δ7.91(d,J=8.6Hz,1H),7.67(d,J=7.9Hz,1H),7.43(t,J=7.7Hz,1H),7.10(d,J=8.6Hz,1H) ,6.72(t,J=7.5Hz,1H),6.63(d,J=8.4Hz,1H),4.20(t,J=7.4Hz,1H),3.83(d,J=9.0Hz,2H),3.53 (ddd, J=17.4, 11.8, 7.5Hz, 1H), 3.43 (d, J=13.5Hz, 1H), 2.99–2.88 (m, 1H), 2.22 (dd, J=11.5, 7.6Hz, 1H), 2.09–1.95(m,2H). 13 C NMR (126MHz, CDCl 3 )δ190.10(s), 171.62(s), 148.39(s), 146.63(s), 144.50(s), 136.08(s), 131.64(s), 129.07(s), 122.58(s), 118.01( s), 117.10(s), 112.97(s), 77.33(s), 77.08(s), 76.83(s), 63.42(s), 61.98(s), 52.84(s), 46.88(s), 31.13( s), 26.21(s), 22.89(s).HRMS(ESI): calcd for C 21 H 20 N 2 O 5 Na[M+Na] + :403.3892,found:403.3896.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com