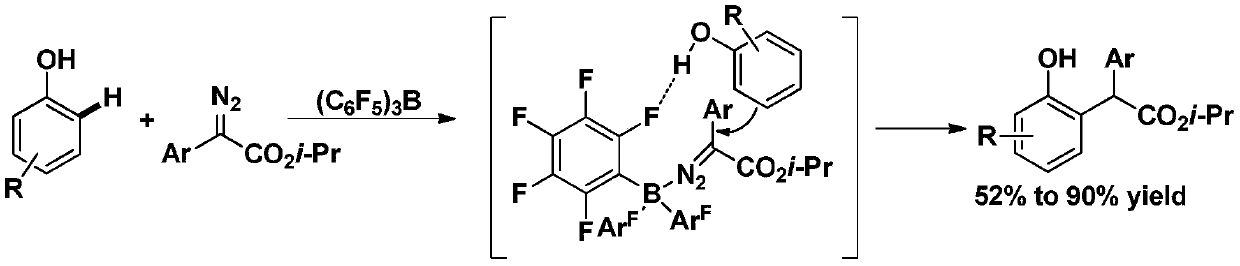
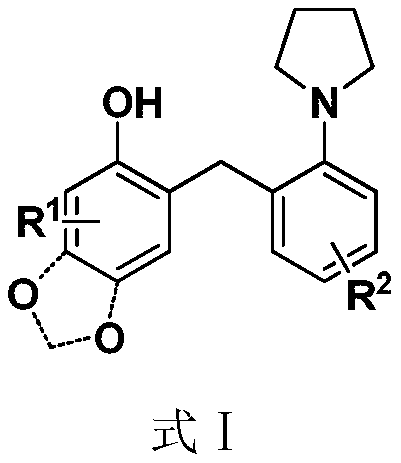
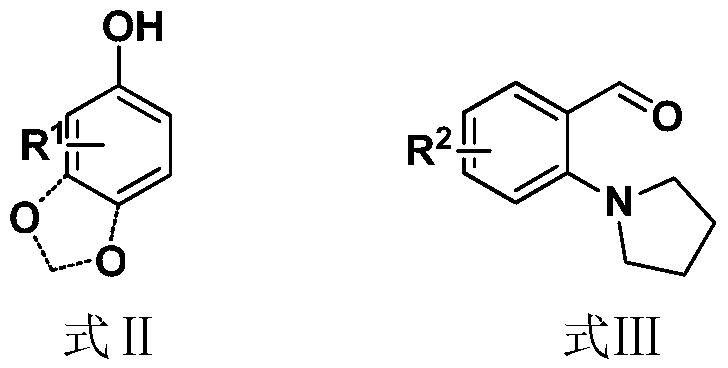
A kind of benzylation synthetic method of phenolic compound
A technology of a phenolic compound and a synthesis method, which is applied in the field of benzylation synthesis of phenolic compounds, can solve the problems of low atom economy, complicated operation and high cost, and achieves complete conversion of raw materials, high reactivity and convenient separation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Weigh compound A 1 (Sesamol) 0.26mmol and Compound B 1 (2-Pyrrolidinylbenzaldehyde) 0.2mmol was placed in a 25mL high-pressure sealed tube, and 2mL of hexafluoroisopropanol HFIP was added, and stirred at room temperature for 12 hours.
[0042] Spotting on a TLC plate, compound B 1 After disappearing, add NaBH to the reaction system 4 (0.4 mmol) was stirred and reacted at room temperature for half an hour, then quenched by adding water, and the system reaction solution was extracted with ethyl acetate.
[0043] First wash the aqueous phase with ethyl acetate for 3 times, combine the two extracts, then add anhydrous sodium sulfate to dry, and concentrate by distillation under reduced pressure. After passing through the silica gel column, a white solid appears after concentration.
[0044] The isolated product was detected and analyzed, and the obtained product was the target product with a yield of 80%.
Embodiment 2
[0046] According to the reaction formula and reaction condition of embodiment 1, sample is spotted on the thin-layer chromatographic plate, compound B 1 After disappearance, the intermediate spiro compound was isolated.
[0047] Set up 4 parallel test groups, add 0.2mmol of the intermediate to the reaction flask, add 0.4mmol of different reducing agents and 2mL of solvent respectively, react at room temperature for 10min, concentrate the reduced product by rotary evaporation, and purify and separate it on a silica gel column.
[0048] The isolated product was detected and analyzed, and the yield of the target product varied with different reducing agents and solvents.
[0049] Table 1 Yield table of intermediate reduction reaction
[0050]
[0051]
[0052] Note: The yield is the isolated yield; the dosage ratio of reducing agent Hantzsch ester to (+ / -)-PA in Group 3 is 26:1.
Embodiment 3
[0055] Raw materials: sesamol, 2-pyrrolidinylbenzaldehyde
[0056] Chemical formula: C 18 h 19 NO 3
[0057] Exact molecular weight: 341.1576
[0058] Molecular weight:
[0059] Structural formula:
[0060] Yield: 80%
[0061] 1 H NMR (500MHz, CDCl 3 )δ10.94(s,1H),7.32(d,J=7.5Hz,1H),7.18(t,J=6.4Hz,2H),7.08(t,J=7.0Hz,1H),6.72(s, 1H),6.39(s,1H),5.80(s,2H),3.81(s,2H),3.17(s,4H),2.06(s,4H); 13 C NMR (126MHz, CDCl 3 )δ150.3, 146.9, 146.3, 140.4, 137.4, 130.7, 127.6, 125.9, 120.2, 119.3, 108.7, 100.7, 99.1, 54.1, 33.8, 23.8.; 13 C NMR (126MHz, CDCl 3 )δ150.3, 146.9, 146.3, 140.4, 137.4, 130.7, 127.6, 125.9, 120.2, 119.3, 108.7, 100.7, 99.1, 54.1, 33.8, 23.8.; high resolution mass spectrometry (ESI) calcd.for C 18 h 19 NO 3 [M+H] + :341.1576, actual value: 341.1580.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com