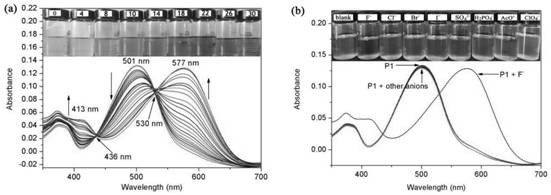
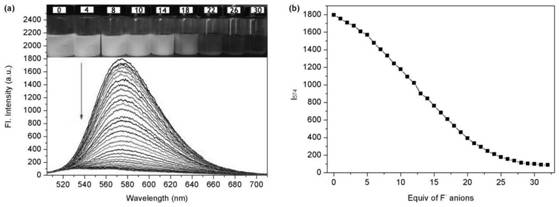
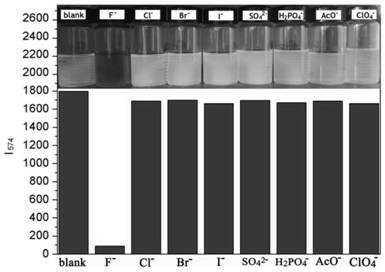
A colorimetric fluorescent probe compound for detecting fluoride ion and its detection method
A technology of fluorescent probes and compounds, applied in the field of analytical chemistry, to achieve the effects of strong fluorescence quenching, good selectivity, and obvious color changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1. Preparation of colorimetric fluorescent probes
[0017] The synthetic route is shown in the following formula:
[0018]
[0019] Compound 3 (1.0 g, 1.44 mmol), zinc powder (0.5 g, 8.0 mmol), ammonium chloride (0.8 mg, 16.0 mmol) were dissolved in 100 mL of tetrahydrofuran. The mixture was stirred at room temperature for 3 h, and the ammonium chloride and zinc dust were removed by filtration. The crude product of the filtrate was purified by silica gel column chromatography to obtain compound 2 (0.76 g, 80%) as a dark brown solid. Compound 2 (0.4 g, 5.0 mmol) and formic acid catalyzed by paraformaldehyde (0.12 g, 4.0 mmol) were dissolved in 100 mL of ethanol and heated under reflux for 12 h. The precipitate was collected by filtration, and the crude product was purified by silica gel column chromatography (dichloromethane: ethyl acetate, 2%) to obtain the colorimetric fluorescent probe P1 of the present invention (0.25 g, 62%, M.p.: 300-302°C). 1H NMR (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com