Formulations of protein molecules comprising iduronate 2-sulfatase
A technology of iduronic acid and protein, applied in the field of protein molecular preparations containing iduronic acid 2-sulfatase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0283] Embodiment 1. A pharmaceutical composition comprising:
[0284] a. A protein molecule comprising:
[0285] i. a first Fc polypeptide; and
[0286] ii. a second Fc polypeptide linked to an enzyme replacement therapy (ERT) enzyme, ERT enzyme variant, or a catalytically active fragment thereof;
[0287] b. Buffers; and
[0288] c. Salt;
[0289] Wherein the pH of the pharmaceutical composition is about 5.5 to 7.0.
Embodiment approach 2
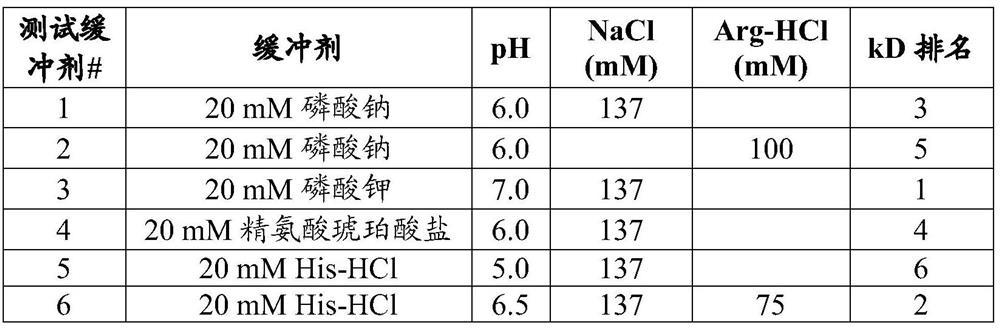
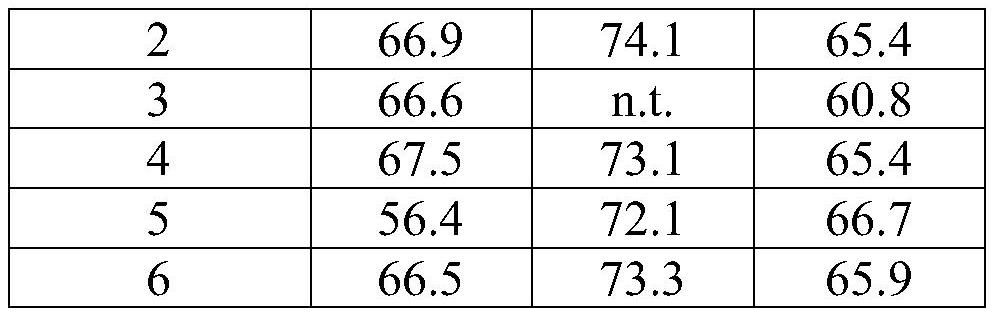
[0290] Embodiment 2. The pharmaceutical composition of embodiment 1, wherein the buffer is selected from the group consisting of phosphate buffer, acetate buffer, arginine buffer, and histidine buffer.
Embodiment approach 3
[0291] Embodiment 3. The pharmaceutical composition of embodiment 2, wherein the phosphate buffer is sodium phosphate buffer or potassium phosphate buffer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com