Controlled release tablet and preparation method thereof
A technology for controlled-release tablets and tablet cores, which can be used in the delivery of pills, pharmaceutical formulations, and medical preparations with inactive ingredients. , the effect of reducing the risk of intestinal obstruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0231] The prescription is as follows:
[0232]
[0233]
[0234] Preparation:
[0235] 1. Preparation of the drug-containing layer: avoid light. Pre-mix nifedipine with other excipients (except magnesium stearate) in the drug-containing layer, add it to a dry granulator and press it into a ribbon, pass through a 16-mesh stainless steel screen to granulate, and finally add magnesium stearate to mix ,spare.
[0236] 2. Preparation of the booster layer: Pre-mix the auxiliary materials of the booster layer (except magnesium stearate), add them into a dry granulator and press them into strips, pass through a 16-mesh stainless steel screen for granulation, and finally add extra stearin Magnesium acid mixed, set aside.
[0237] 3. Tablet compression: keep away from light. Use a double-layer tablet press machine to compress the double-layer tablet, the die size is 6mm round tablet, first fill 82.5mg of drug-containing layer material, pre-press, then fill 41.3mg of booster l...
Embodiment 2
[0241] The prescription is as follows:
[0242]
[0243]
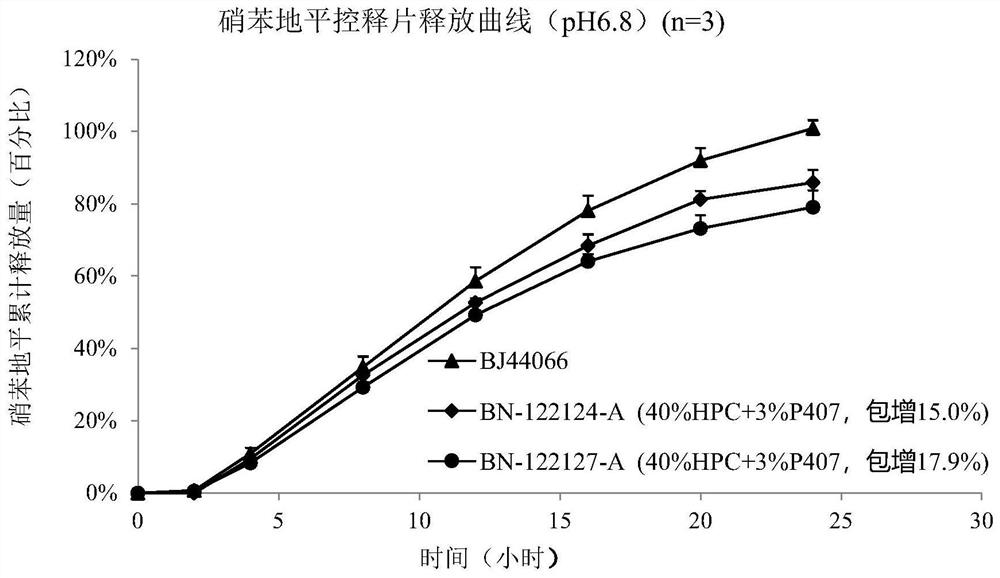
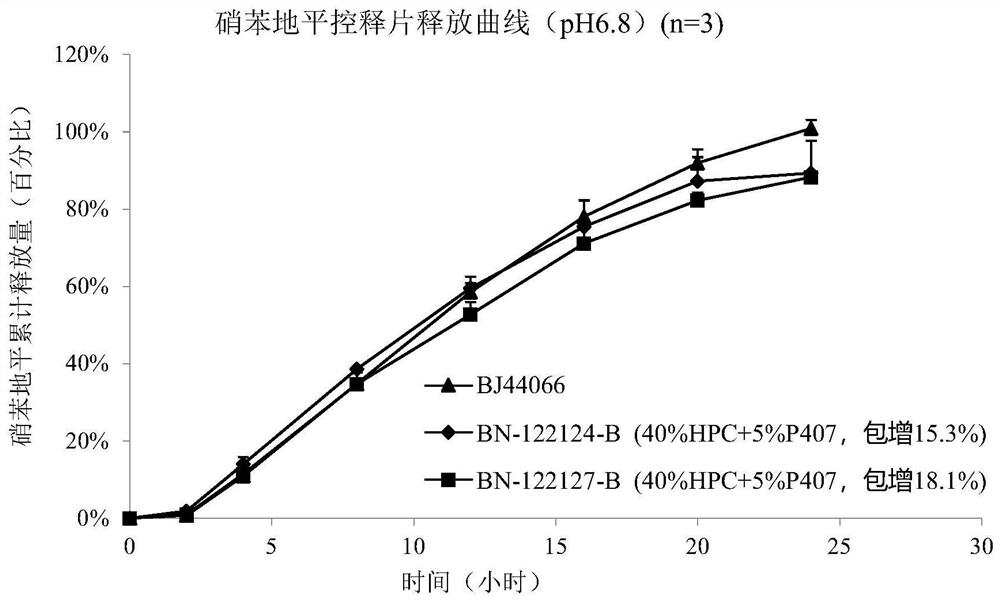
[0244] Preparation method: The preparation method of the double-layer tablet core is the same as in Example 1, and the weight of each double-layer tablet core is 123.8 mg. A coating pan was used to coat the double-layer tablet cores with the above-mentioned controlled-release coating solution to a weight gain of 15.3 wt% and 18.1 wt%, and a drug release pore size of 0.5 mm. The release measurement method is the same as in Example 1. The specification of the controlled-release tablet in this embodiment is 30 mg / tablet, a round tablet with a diameter of 6 mm. figure 2 Be the nifedipine controlled-release tablet release curve in embodiment 2. Not broken.
Embodiment 3
[0256] The prescription is as follows:
[0257]
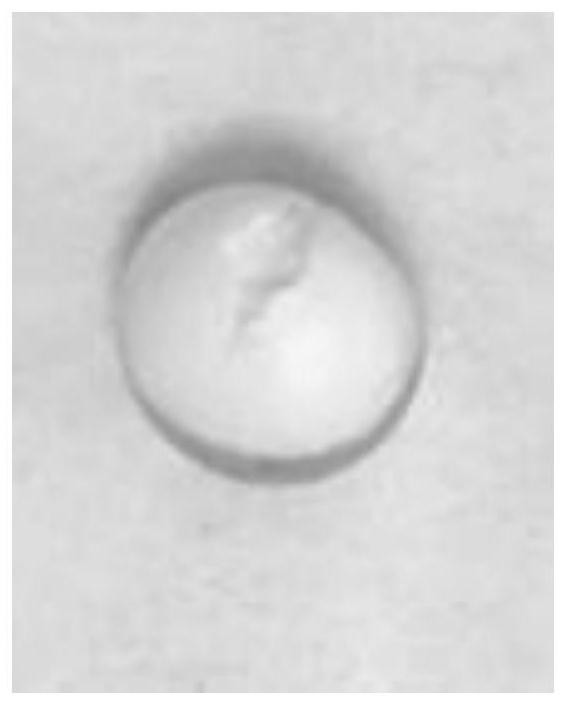
[0258] Preparation method: The preparation method of the double-layer tablet core is the same as that in Example 1. The weight of each double-layer tablet core is 165 mg, and the weight of the booster layer is 55.0 mg / tablet. A coating pan is used to coat the double-layer tablet core with the above-mentioned controlled-release coating solution, the weight gain of the coating is 15.0%, and the drug release aperture is 0.5mm. The release measurement method is the same as in Example 1. The specification of the controlled-release tablet in this embodiment is 30mg / tablet, and the circular tablet has a diameter of 7mm. The observation chart of whether the controlled-release membrane is broken during the release process can be found in Figure 24 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap