Method for evaluating in-vivo and in-vitro correlation of theophylline sustained-release tablets
A sustained-release tablet and correlation technology, which is applied in the field of evaluating the in vitro and in vivo correlation of theophylline sustained-release tablets, can solve the problems of not being able to evaluate the pharmacokinetic characteristics in vivo well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 2
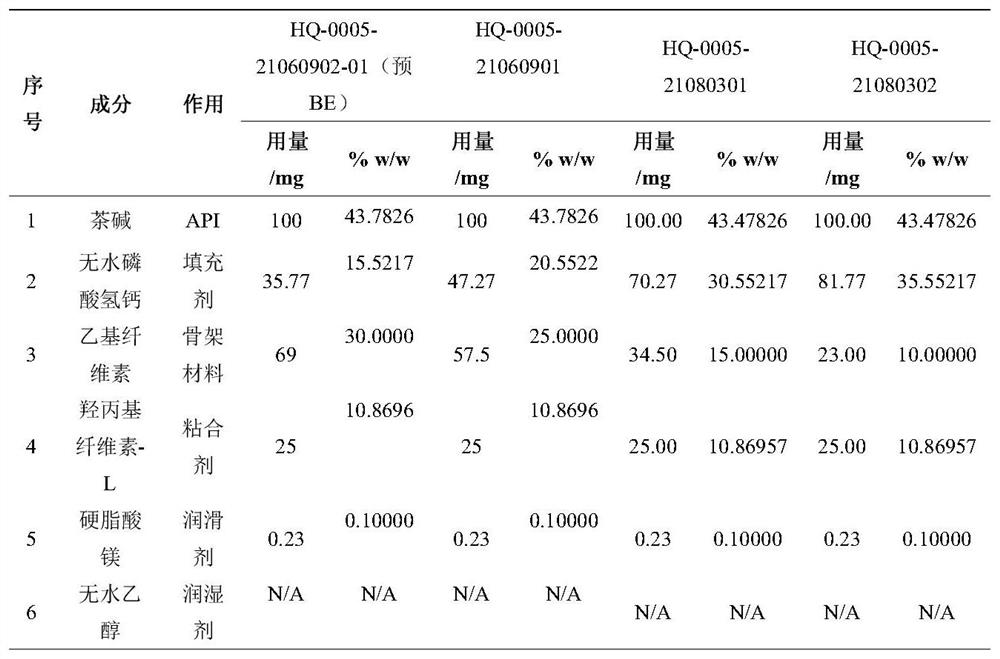
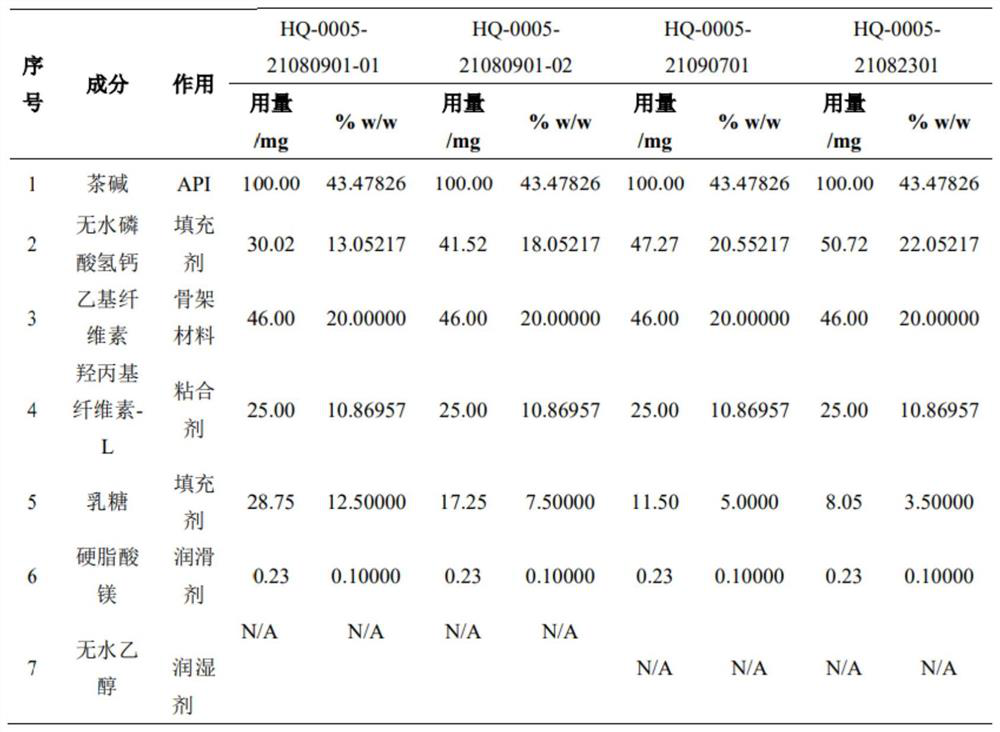
[0043] Embodiment 2 The changes of theophylline sustained-release tablets observed by contrasting the disintegration phenomenon of theophylline sustained-release tablets in pH4.5+pH6.0-xanthan gum+glass beads medium by comparing different prescriptions. The formulations of different batches of self-developed preparations are HQ-0005-21082301 (20% EC+3.5% lactose) medium containing 1% xanthan gum, HQ-0005-21090701 (20% EC+5% lactose) medium containing 1 % Xanthan gum, HQ-0005-21080901-02 (20% EC+7.5% lactose) medium containing 1% xanthan gum, pilot product 21012401 batch (mid-term) medium containing 1% xanthan gum, HQ-0005-21060902 (EC dosage 30%) The medium contains 1% xanthan gum. When 1% xanthan gum is added to the glass bead medium (pH4.5, pH6.0), the HPMC prescription self-developed preparation has a greater degree of softening and abrasion, while other EC and EC+lactose self-developed preparations basically have no Variety.
Embodiment 3
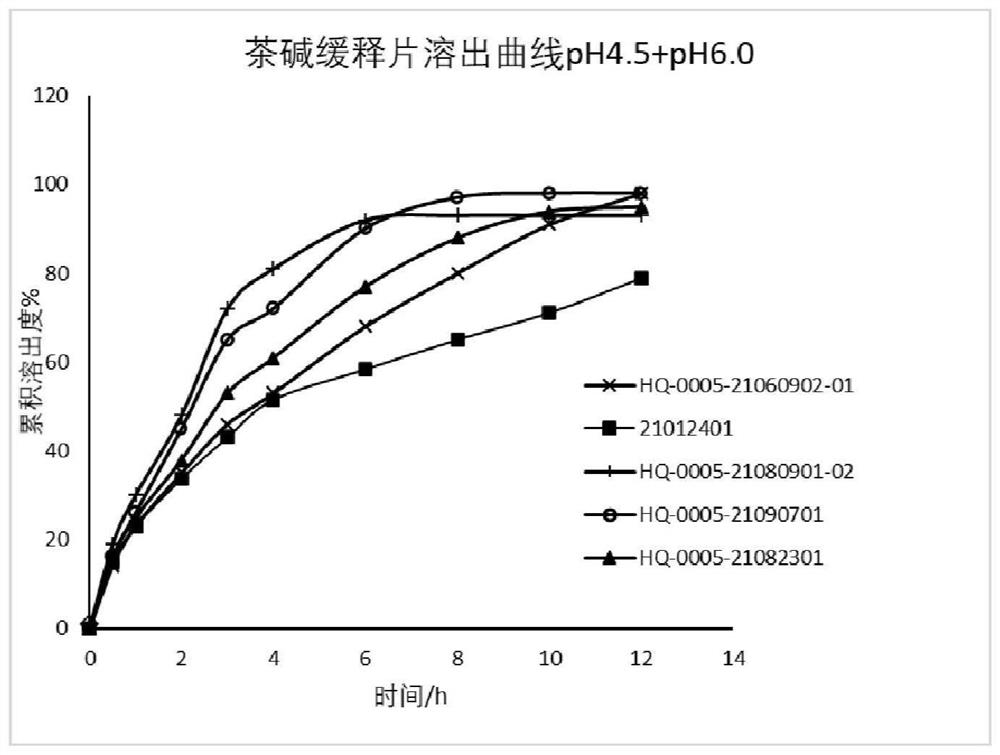
[0044] Embodiment 3 by contrasting the release properties of theophylline sustained-release tablets of different prescriptions, such as image 3Shown, the release performance of theophylline sustained-release tablets in pH4.5+pH6.0 medium using USP III method. The formulations of different batches of self-developed preparations are HQ-0005-21060902-01 (30% EC), 21012401 (HMPC), HQ-0005-21080901-02 (20% EC+7.5% Lactose), HQ-0005- 21090701 (20% EC+5% Lactose), HQ-0005-21082301 (20% EC+3.5% Lactose), RLD 89A26P. By comparing the dissolution curves of the self-developed preparations of different prescriptions in pH4.5+pH6.0 media, the dissolution curves of the self-developed preparations formulated by HPMC are closest to those of the reference preparation (pre-BE results show that Cmax is on the high side); 20% EC The dissolution curve of the self-developed preparation with +5% Lactose prescription is relatively close to that of the reference preparation.
Embodiment 4
[0045] Embodiment 4 by contrasting the release properties of theophylline sustained-release tablets of different prescriptions, such as Figure 4 Shown, the release performance of theophylline sustained-release tablets in pH4.5+pH6.0 medium by flow cell method. The formulations of different batches of self-developed preparations are HQ-0005-21060902-01 (30% EC), 21012401 (HMPC), HQ-0005-21080901-02 (20% EC+7.5% Lactose), HQ-0005- 21090701 (20% EC+5% Lactose), HQ-0005-21082301 (20% EC+3.5% Lactose), RLD 89A26P. By comparing the dissolution curves of self-developed preparations with different prescriptions in pH4.5+pH6.0 media, the results showed little difference, but there was a big difference with the reference preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com