A detection kit for Marfan syndrome based on fbn1 gene insertion mutation
A detection kit and insertion mutation technology, applied in biological testing, microbial determination/inspection, measurement devices, etc., can solve problems such as difficult early diagnosis, large individual differences, etc., achieve simple operation, good accuracy, and reduce disease Effects of Rate and Mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. FBN1 gene c.5688-5689 (p.Arg1897Glufs) insertion mutation detection kit The components of the kit described in Example 1 are described in Table 1 below.
[0042] Table 1. Kit Components
[0043]
Embodiment 2
[0044] Example 2. Patient / Carrier Validation Experiment
[0045] 1. Sample collection and clinical data collection
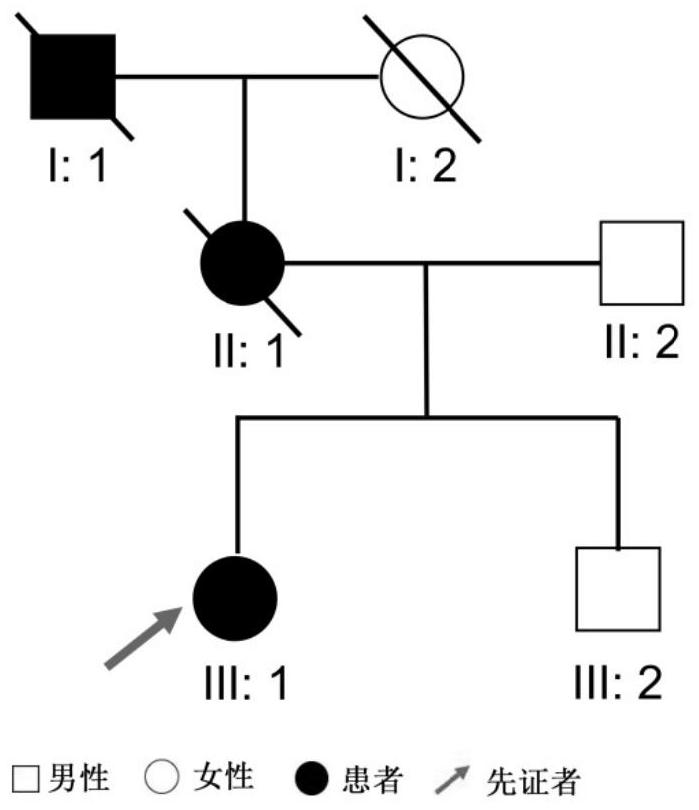
[0046] On the premise that the proband and his family voluntarily signed the informed consent, a whole blood sample of about 5 mL was drawn in Shenzhen Eye Hospital and stored in a low temperature refrigeration condition. . This study was approved by the Ethics Committee of Shenzhen Eye Hospital.
[0047] 2. Preparation of Genomic DNA
[0048] Whole genome DNA was extracted from human whole blood EDTA anticoagulant samples using commercially available DNA extraction kits, and the concentration and purity of DNA were detected.
[0049] 3. PCR primer design
[0050] According to the DNA sequence of the FBN1 gene in the NCBI database (https: / / www.ncbi.nlm.nih.gov / ), primers were designed upstream and downstream of the mutation position of the FBN1 gene. The specific primer sequences are shown in Table 2.
[0051] Table 2. Primer Information
[0052]
[005...
Embodiment 3
[0079] Example 3. Repeatability detection
[0080] Using the method described in Example 2 of the present invention, the blood samples of 1 patient with Marfan syndrome and 1 healthy person were repeatedly detected three times, and the detection results are shown in Table 5 below.
[0081] Table 5. Repeatability test results
[0082] sample patient healthy person the first time positive feminine the second time positive feminine the third time positive feminine
[0083] The above detection results show that the sites and detection primers described in this application have good repeatability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com