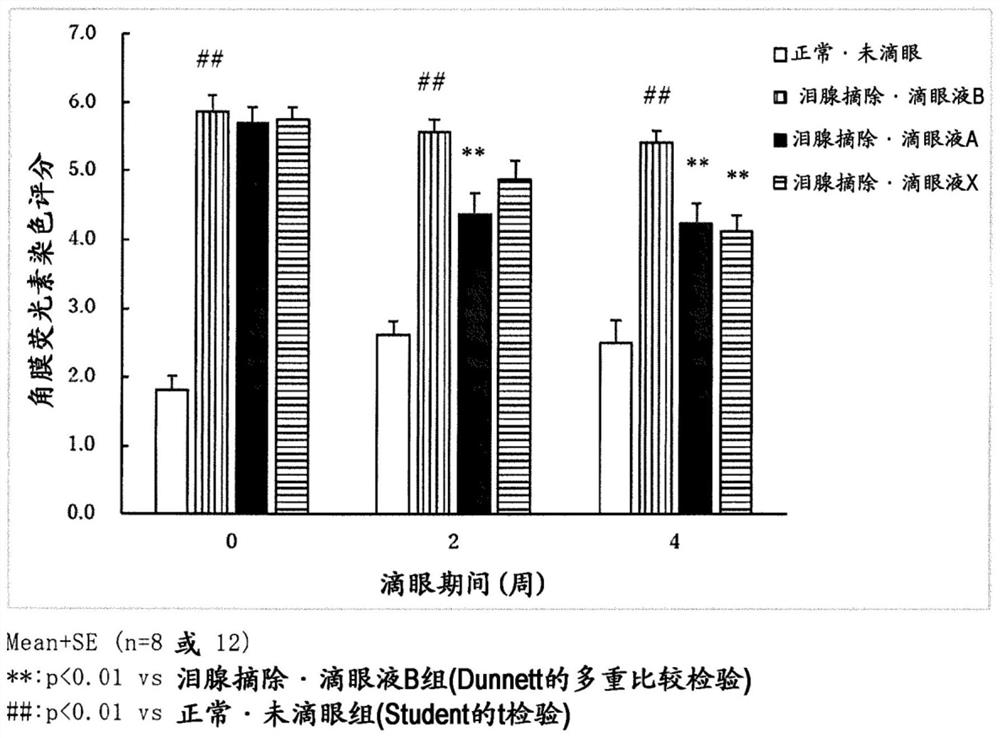
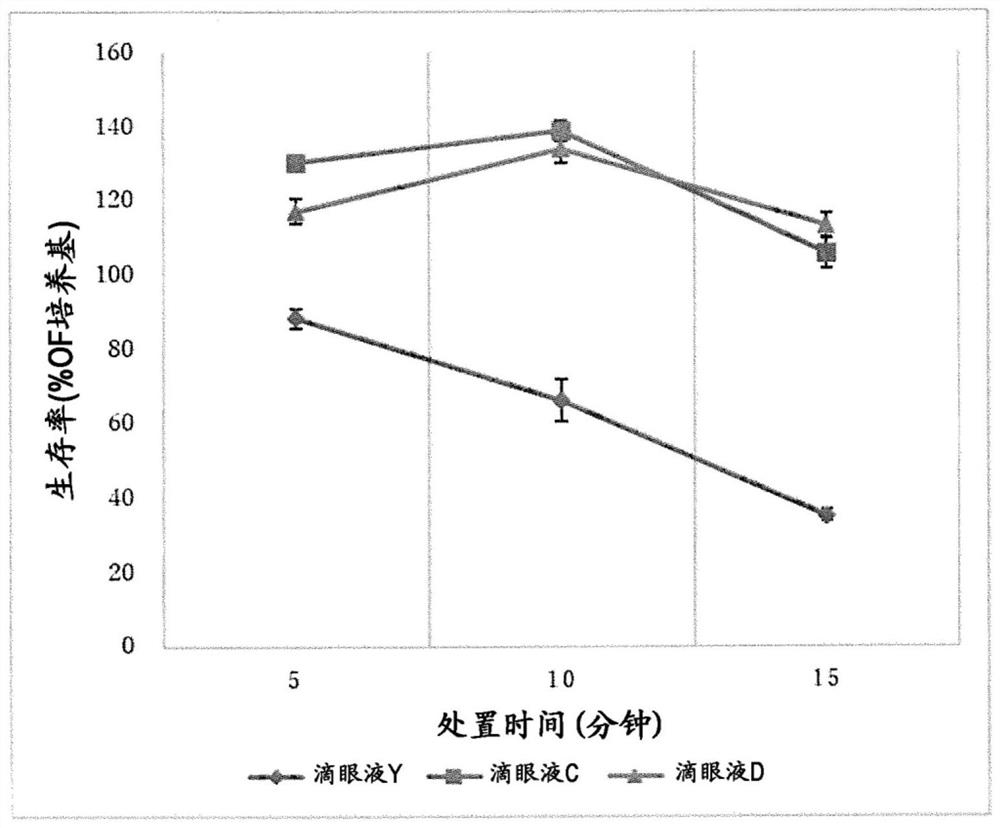
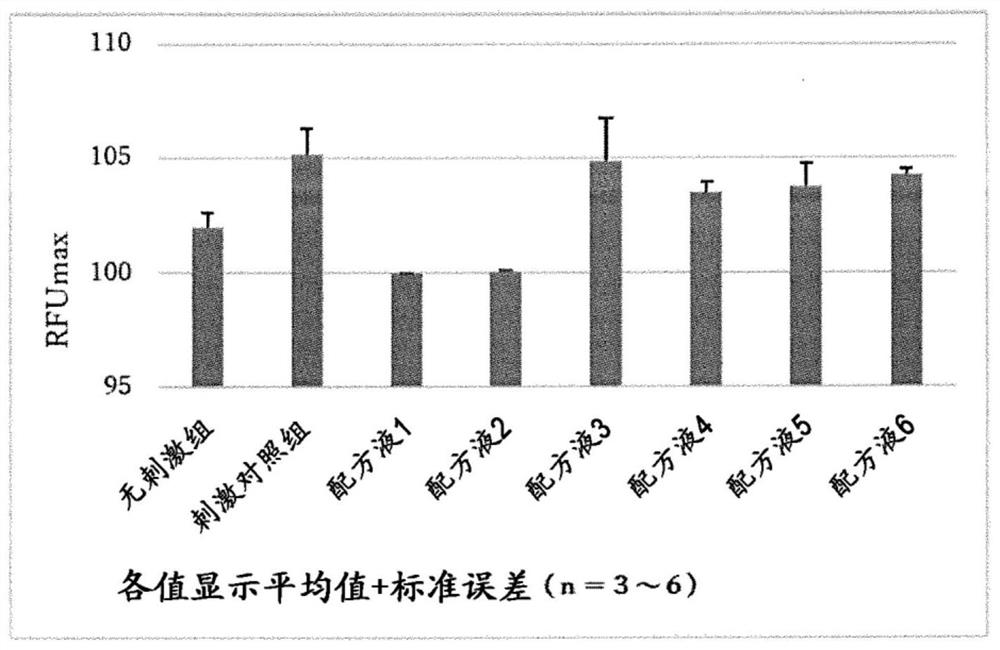
Aqueous ophthalmic composition containing diquafosol or salt thereof, and polyvinylpyrrolidone
A polyvinylpyrrolidone and water-based composition technology, applied in the field of water-based ophthalmic compositions, can solve the problems of inability to obtain expected effects, poor compliance with eye drops, unable to obtain sufficient therapeutic effects, etc., and achieve excellent preservation efficacy and compliance with eye drops. The effect of sexual improvement, strong dry eye treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0151] The results of pharmacological tests and preparation examples are shown below, but these examples are for better understanding of the present invention and do not limit the scope of the present invention.
[0152] [test 1]
[0153] Using normal male white rabbits, the temporal change in the amount of tears after instilling the present composition was evaluated.
[0154] (Sample preparation method)
[0155] Eye drops 1:
[0156] According to the formulation table shown in Table 1, eye drop 1 was prepared. That is, diquafosol sodium (9 g), sodium hydrogen phosphate hydrate (0.6 g), edetate sodium hydrate (0.03 g) and sodium chloride (1.35 g) were dissolved in sterilized purified water to make It was 50 mL, resulting in a 6-fold concentrate. In addition, after mixing 10 mL of the 6-fold concentrated solution with 5 mL of sterilized purified water, a pH regulator was appropriately added to adjust the pH to 7, and 20 mL of sterilized purified water was added to obtain a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com