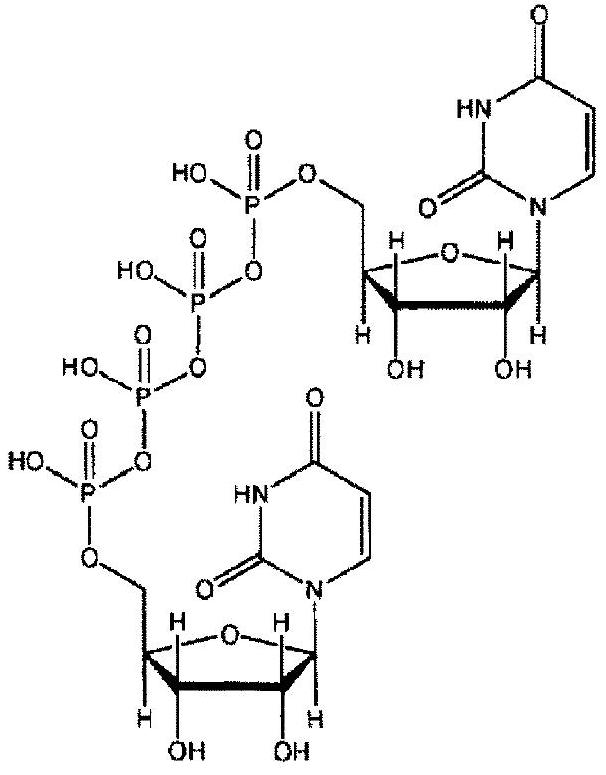
Ophthalmic composition containing diquafosol or salt thereof, vinyl-based polymer and cellulose-based polymer
A diquafosol, vinyl-based technology, applied to containing diquafosol or a salt thereof, can solve the problems of poor eye drop compliance, patients unable to obtain expected results, frequent eye drop, etc., to improve eye drop compliance , strong dry eye treatment effect, the effect of reducing the number of eye drops
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
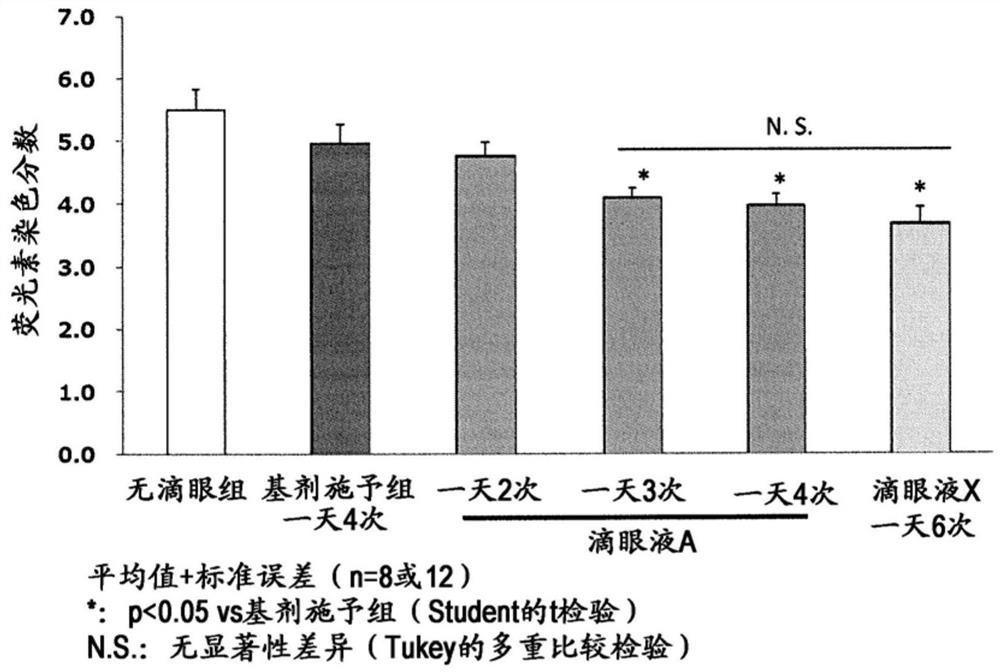
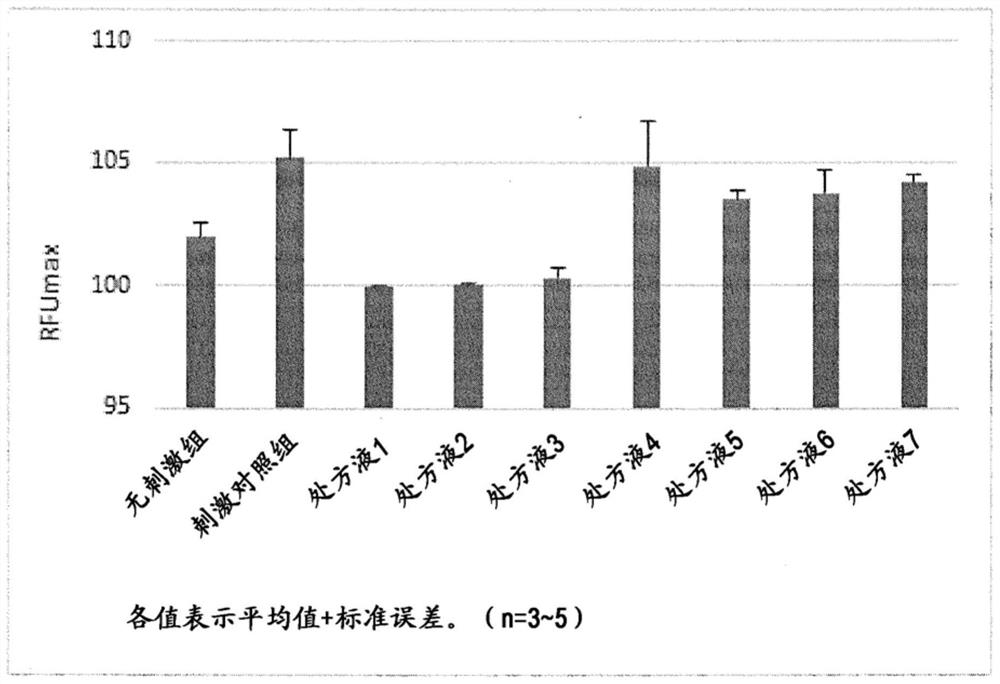
[0101] The results of pharmacological tests and preparation examples are shown below, but these examples are for better understanding of the present invention and do not limit the scope of the present invention.
[0102] [test 1]
[0103] Normal male white rabbits were used to evaluate changes over time in the amount of tears after instilling the present composition.
[0104] (drug preparation method)
[0105] Eye drops 1:
[0106] According to the prescription table shown in Table 1, eye drop 1 (in Table 1, the unit is g / 100mL) was prepared. That is, diquafosol sodium (9 g), sodium hydrogen phosphate hydrate (0.6 g), edetate sodium hydrate (0.03 g) and sodium chloride (1.35 g) were dissolved in sterilized purified water to obtain 50 mL of 6 times thick liquid. In addition, after mixing 10 mL of the 6-fold concentrated solution and 5 mL of sterilized purified water, PVP K30 (1.2 g) was dissolved, and then, a pH adjuster was added appropriately to adjust the pH to 7, and 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com