Signal analysis of movement of reference electrode of catheter in coronary sinus vein
A catheter and electrode technology, applied in the field of signal processing, can solve problems such as improving the stability of electrodes/catheters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
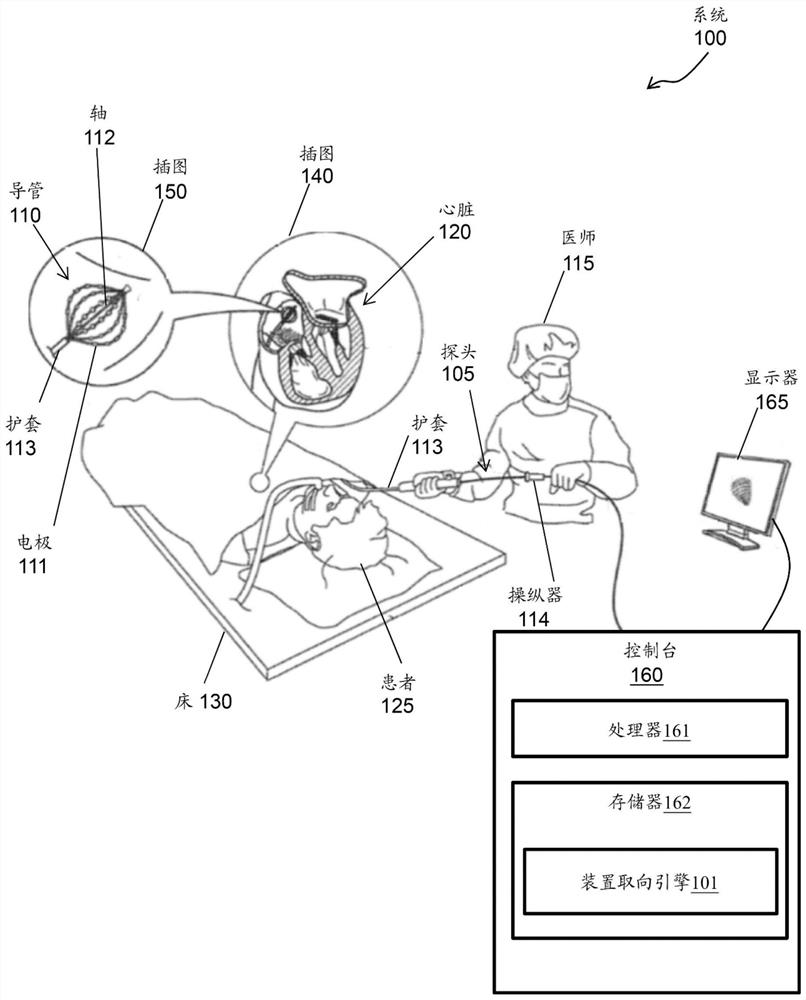
[0020] A method and system for signal processing are disclosed herein. More specifically, the present invention relates to signal analysis of movement of a reference electrode of a catheter in a coronary sinus (CS) vein.
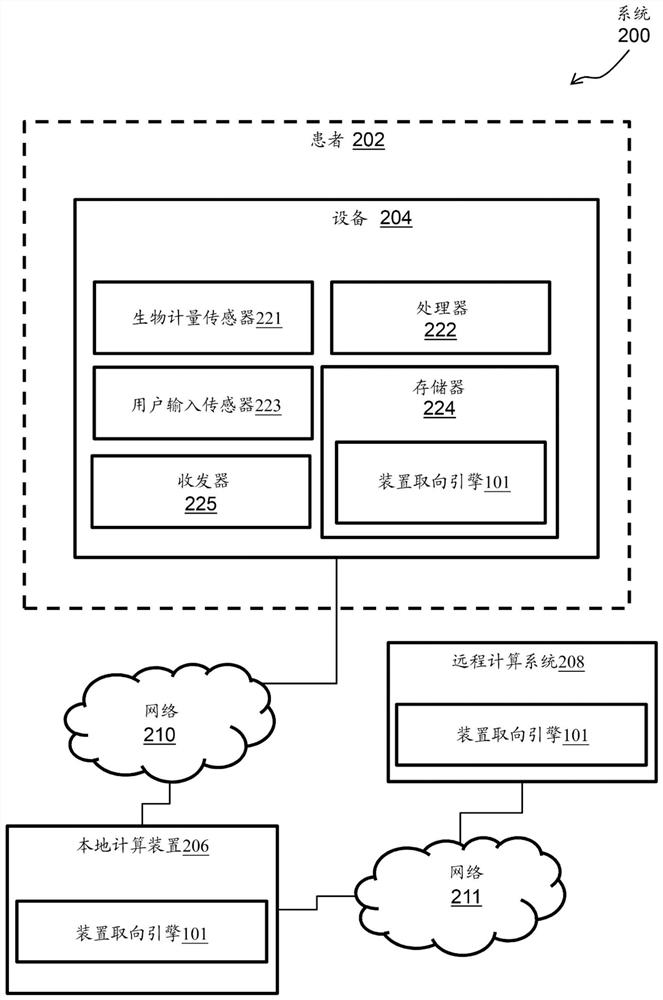
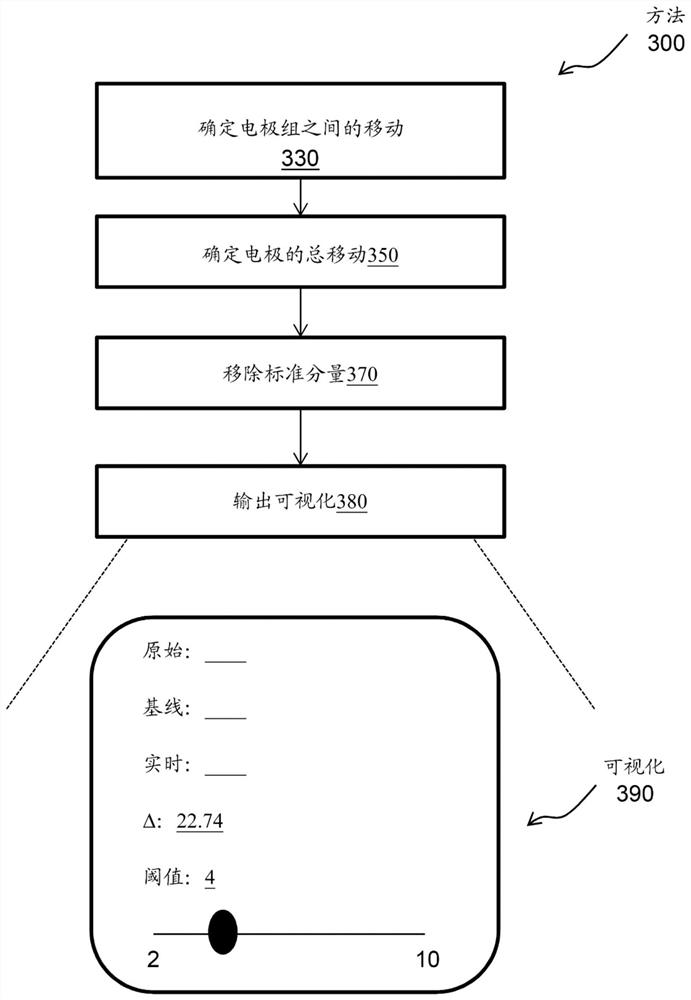
[0021] For example, a device-oriented engine is processor-executable code or software that must be rooted in the process operations performed by the medical device device as well as in the processing hardware of the medical device device. According to an exemplary embodiment, the device orientation engine may include a machine learning / artificial intelligence (ML / AI) algorithm. The device orientation engine tracks the movement of the CS catheter within the CS while mapping the CS. For example, the device orientation engine tracks the movement of the CS catheter along the axial axis of the CS (which can affect the stability of the map's reference), and tracks deviations from the acquired reference position at the beginning of the mapping.
[0022] Technical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com