Genetic pharmacopoeia for comprehensive functional analysis of human cancer
A cancer and functional technology, used in the introduction of foreign genetic material, analytical materials, drug combinations, etc. using vectors, which can solve problems such as insufficiency and ineffective treatment cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
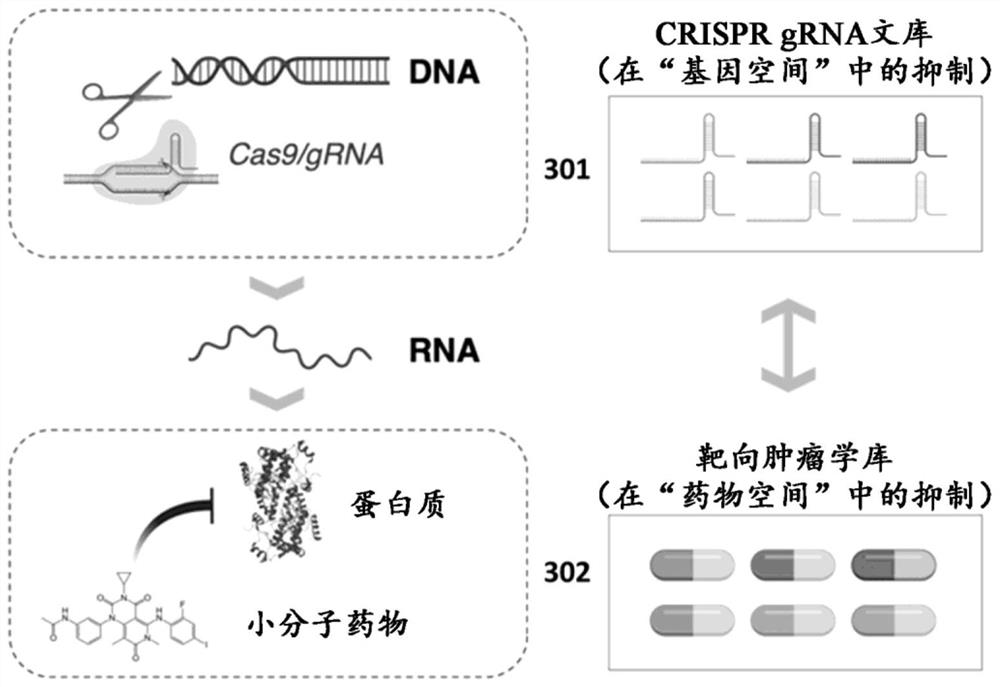
[0126] (1) A method of treating cancer in a subject in need thereof, said method comprising administering to said subject a therapeutic molecule selected from a library of therapeutic molecules; wherein said therapeutic molecule is selected by a method comprising the steps of : modifying cancer cells from said subject to knock down or knock out the function of a plurality of genes, each gene in said plurality of genes encoding a protein target of a therapeutic molecule in said therapeutic molecule library, wherein if said The therapeutic molecule is selected if the therapeutic molecule knocks down or knocks down the function of a gene encoding a protein target of the selected therapeutic molecule impairs cancer cell viability.
[0127] (2) The method according to said embodiment 1, wherein said library of therapeutic molecules comprises at least 1, at least 2, at least 3, at least 4, at least 5, at least 10 or At least 20 healing agents.
[0128] (3) The method according to e...
Embodiment 1
[0569] Example 1: Genetic Pharmacopoeia
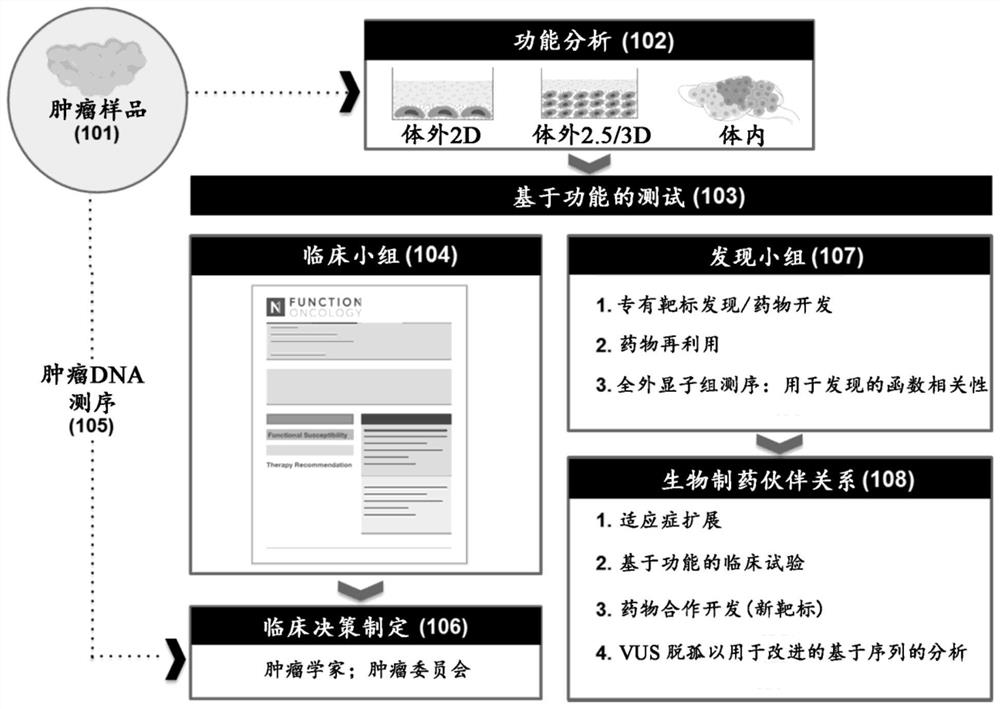
[0570] A drug library comprising the molecularly targeted tumor drugs of Table 2B was generated. The drug library is regularly updated to include additional tumor-targeting drugs as they are identified. A genetic pharmacopoeia was generated to represent the genetic targets of the drug library (Table 5B).
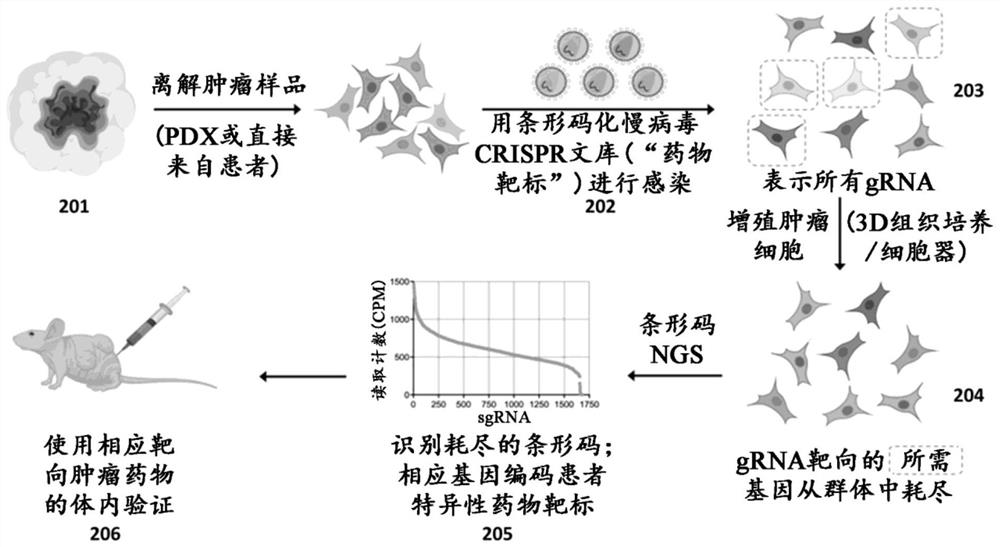
[0571] Libraries of gene regulators containing guide RNA (gRNA) sequences associated with each gene target were designed. As shown in Table 6A, five potential gRNA sequences were designed for each tumor drug target to generate gRNA sequences with SEQ ID NOS: 1-1525. A library of gene regulators is constructed to comprise at least one gRNA sequence selected from SEQ ID NOS: 1-1525. Libraries were constructed using viral delivery methods (adenovirus for Cas nuclease delivery and lentivirus for gRNA delivery) in a format compatible with use in primary cancer cells.
Embodiment 2
[0572] Example 2: Cancer Functional Susceptibility Analysis
[0573] A method is performed to determine the functional susceptibility of cancer cells in a patient to one or more interferors that mimic the effects of the tumor-targeting drugs identified in Example 1. Libraries comprising at least one gRNA sequence selected from SEQ ID NOS: 1-1525 and associated gene editing agent(s) (e.g., RNA-guided nucleases) are delivered to patient-derived primary cancer cells , in order to genetically modify cancer cells. Delivery of Cas nuclease and gRNA via lentivirus. In this example, genetic modification was performed by gene editing using a CRISPR-based approach. The modified cancer cells proliferate in vivo, however, the method can be used in an in vitro environment that mimics the in vivo environment. The effect of each gene edit was assessed by screening the modified cancer cells in pools or arrays. Next-generation sequencing techniques are performed to determine the effect of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com