Substituted pyrrolopyrimidine compound and application thereof
A technology of compounds and compositions, applied in the field of substituted pyrrolopyrimidine compounds and their preparation, prevention and treatment of diseases mediated by C-Kit and/or PDGFR, capable of solving problems such as fast metabolism, achieving slow metabolism and high-quality drugs Kinetic properties, long half-life effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
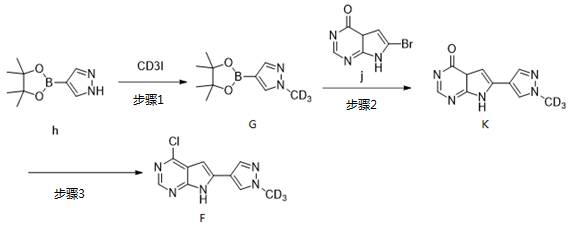
[0025] Example 1: Preparation of Compound 1
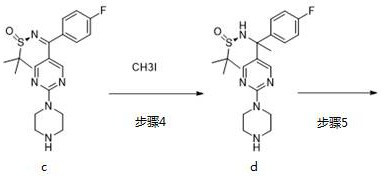
Step 1: Synthesis of Compound c
Add compound a (2.86 g, 10 mmol) and anhydrous THF (200 mL) to a single product bottle, stir the solution, add compound b (2.42 g, 20 mmol) and tetraethyl titanate (6.83 g, 30 mmol) , the temperature was raised to 70 °C under nitrogen protection, and stirred overnight. After monitoring the reaction by TLC, water (100 mL) was added, extracted with ethyl acetate (300 mL) three times, the organic phases were combined, washed twice with water, twice with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, and concentrated. The product was separated by column chromatography to obtain 2.43 g of a yellow solid. Yield: 62.5%.
[0026] MS: m / z (ES): 390.5 [M+1].
[0027] Step 2: Synthesis of Compound D
Magnesium powder (280 mg, 11.6 mmol) was added to a 50 mL double-necked flask, evacuated and protected by nitrogen, ether (10 mL) was added, and deuterated iodomethane CD3I (1.44...
Embodiment 2
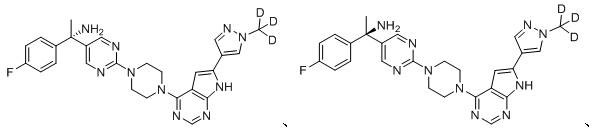
[0035] Example 2: Preparation of Compound 2
100 mg of compound 1 was taken and separated by chiral HPLC to obtain 40 mg of white solid, which was the isomer compound 2.
[0036] MS m / z (ES): 502.5 [M+1].
[0037] 1 H NMR (300MHz, DMSO -d6 ): 8.59 (m, 2H), 8.21 (m, 1H), 7.91-7.96 (m, 2H), 7.19-7.28 (m, 4H), 6.41(s, 1H), 5.10 (t, 2H), 5.01( s, 1H), 3.95 (m, 3H), 3.26 (m, 4H), 3.18-3.21 ((m, 4H)).
Embodiment 3
[0038] Example 3: Preparation of Compound 3
100 mg of compound 1 was taken and separated by chiral HPLC to obtain 40 mg of white solid, which was the isomer compound 3.
[0039] MS m / z (ES): 502.5 [M+1].
[0040] 1 H NMR (300MHz, DMSO -d6 ): 8.59 (m, 2H), 8.21 (m, 1H), 7.91-7.96 (m, 2H), 7.19-7.28 (m, 4H), 6.41(s, 1H), 5.10 (t, 2H), 5.01( s, 1H), 3.95 (m, 3H), 3.26 (m, 4H), 3.18-3.21 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com