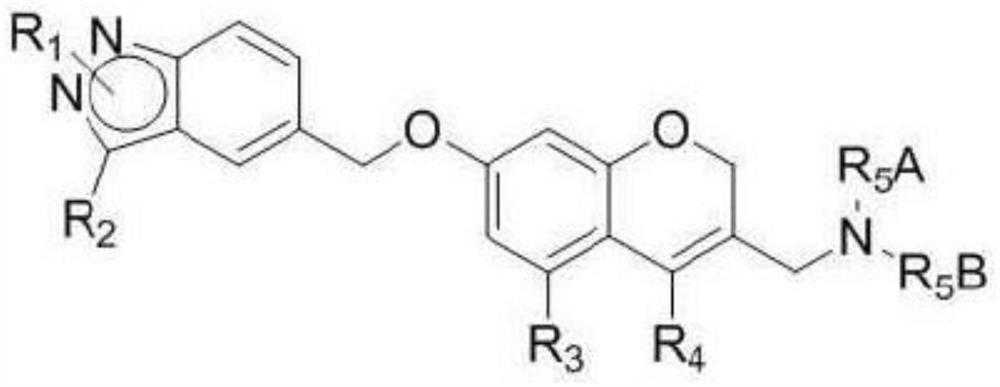
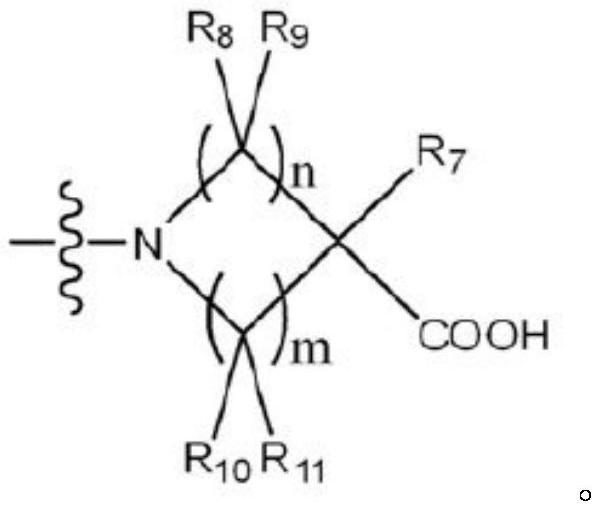
Sphingosine-1-phosphate receptor agonist, preparation method thereof and pharmaceutical composition containing same as active ingredient
A compound and pharmaceutical technology, applied in the field of sphingosine-1-phosphate receptor agonist, its preparation and pharmaceutical composition containing it as an active ingredient, can solve the problem of decreased lymphocyte infiltration and achieve therapeutic or preventive immunity Effects of adjustment disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1-1
[0104] Preparation Example 1-1: Synthesis of 7-hydroxy-2H-chromene-3-carbaldehyde
[0105] The title compound was obtained according to the method described in International Patent Publication No. 2010-064707.
[0106] NMR: 1 H-NMR (400 HMz, CDCl 3 );δ9.51(s,1H),7.40(m,5H),7.19(s,1H),7.11(d,1H),6.60(dd,1H),6.49(d,1H),5.10(s, 2H),5.00(s,2H)
preparation example 1-2
[0107] Preparation Example 1-2: Synthesis of (3-chloro-1-isopropyl-1H-indazol-5-yl)-methanol
[0108] The title compound was obtained according to the method described in Korean Registered Patent No. 10-1939657.
[0109] NMR: 1 H-NMR (400HMz, CDCl3); δ 7.64 (s, 1H), 7.43 (m, 2H), 4.79 (m, 3H), 1.83 (br s, 1H), 1.56 (d, 6H)
preparation example 1-3
[0110] Preparation Example 1-3: Synthesis of 7-(3-chloro-1-isopropyl-1H-indazol-5-ylmethoxy)-2H-chromene-3-carbaldehyde
[0111] (3-Chloro-1-isopropyl-1H-indazol-5-yl)-methanol (383 mg, 1.70 mmol) obtained from Preparation Example 1-2 and 7-hydroxy- 2H-chromene-3-carbaldehyde (300 mg, 1.70 mmol) was dissolved in toluene (10 mL), and tributylphosphine (BuP 3 , 291 mg, 1.44 mmol) and 1,1'-(azodicarbonyl)dipiperidine (ADD, 363 mg, 1.44 mmol). After stirring the mixture at room temperature for 18 hours, an excess of hexane was added thereto. The mixture was filtered, and the filtrate was distilled under reduced pressure. The residue was purified by column chromatography to give the title compound (320 mg, 49%).
[0112] NMR: 1 H-NMR (400 HMz, CDCl 3 );δ9.52(s,1H),7.71(s,1H),7.47(dd,J=1.6Hz,1H),7.44(d,1H),7.21(s,1H),7.14(d,1H) ,6.62(dd,J=2.4Hz,1H),6.52(d,1H),5.16(s,2H),5.03(s,2H),4.83-4.76(m,1H),1.57(d,6H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com