Application of leonurine in preparation of medicine for preventing and treating non-vascular dementia or infectious central nervous injury
A technology of leonurine and central nervous system, which is applied in the field of medicine and can solve the problems of unseen neuron effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
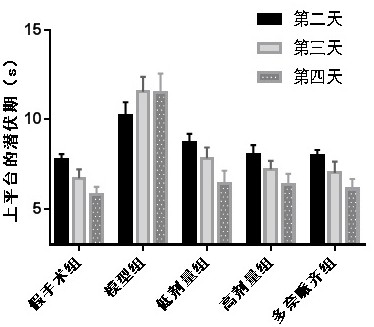
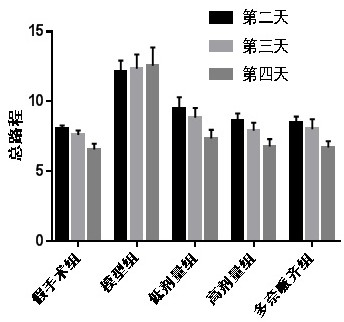
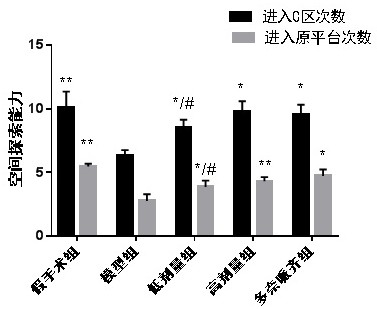
[0041] Example 1: Leonurine improves spatial learning and memory ability and spatial exploration ability of AD mice
[0042] Thirty mice were randomly divided into 5 groups with 6 mice in each group, which were (1) sham-operated group; (2) model group; (3) low-dose group (oral Leonurine 8 mg / kg); (4) high-dose group Group (oral Leonurine 80mg / kg); (5) Donepezil group (Oral donepezil 1.5mg / kg).
[0043]Dissolve amyloid Aβ1-42 with sterile physiological saline at a concentration of 500 μmol / L, and incubate in a 37°C incubator for 5 days before use. Mice were anesthetized by intraperitoneal injection of 10% chloral hydrate, and fixed on the brain stereotaxic apparatus. After routine disinfection, the middle skin of the top of the mouse head was incised, and the midline and bregma of the skull were cleaned and fully exposed, and the skull surface marker points ( The anterior fontanelle is zero point, the posterior fontanelle is 3.5mm, and the midline is 2.5mm), and a hole is dril...
Embodiment 2
[0057] Example 2: The effect of leonurine on the deposition of senile plaques in the cortex and hippocampus of AD mice
[0058] The senile dementia mouse model described in Example 1 was used, and the mice were killed by decapitation after administration, and the brain tissue was quickly taken out. The brain tissue was rinsed in ice-cold saline, dried with filter paper, and weighed. The protein lysis solution (containing PMSF) of twice the tissue weight was fully lysed with a homogenizer on ice; after lysis for 30 min, it was transferred to a centrifuge tube, centrifuged at 4°C and 12000 rpm for 5 min, and the supernatant was collected in a 1.5 ml centrifuge tube. middle. Measure the protein concentration with a protein quantifier, then adjust the protein concentration to 20 μg / μL with RIPA lysis buffer, and mix the two in a ratio of 1 μL protein loading buffer (5X) per 4 μL protein sample, 100 °C or boiling water. Heat in the bath for 3-5min and store in -20℃ refrigerator. ...
Embodiment 3
[0066] Example 3: Effects of leonurine on acetylcholine (Ach) and brain-derived nerve growth factor (BDNF) in AD mice brain
[0067] The senile dementia mouse model described in Example 1 was used, and the mice were killed by decapitation after administration, and the brain tissue was quickly taken out. The brain tissue was rinsed in ice-cold saline, dried with filter paper, and weighed. The protein lysis solution (containing PMSF) of twice the tissue weight was fully lysed with a homogenizer on ice; after lysis for 30 min, it was transferred to a centrifuge tube, centrifuged at 4°C and 12000 rpm for 5 min, and the supernatant was collected in a 1.5 ml centrifuge tube. middle. The contents of Ach and BDNF were detected by ELISA kit.
[0068] The results are shown in Table 6. Compared with the sham-operated group, the contents of Ach and BDNF in the brains of the mice in the model group were significantly reduced, while both the high and low doses of leonurine and the positive...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap