Microwell plate for containing plurality of samples
A microplate and sample technology, applied in the field of microplate, can solve the problems of reducing experimental output and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
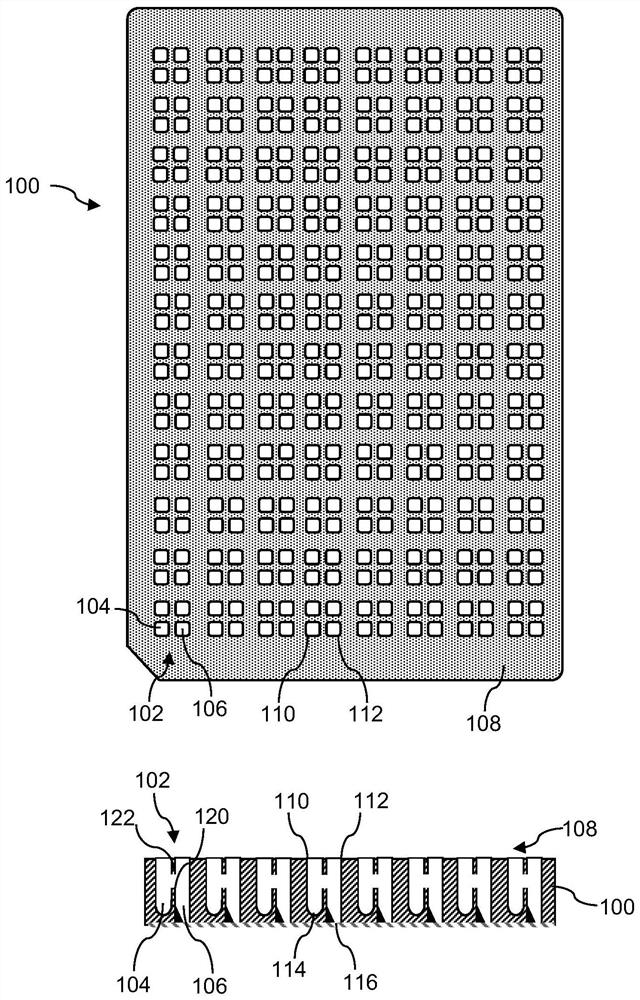
[0031] figure 1 A schematic top view and corresponding cross-sectional view of the microplate 100 are shown. Microplate 100 includes 192 sample chambers 102 arranged in an 8 by 24 grid. Each cavity 102 includes a donor compartment 104 and an acceptor compartment 106 . Thus, the microplate 100 includes 384 compartments 104 , 106 . Alternatively, the microplate 100 may have additional or fewer sample cavities 102 .
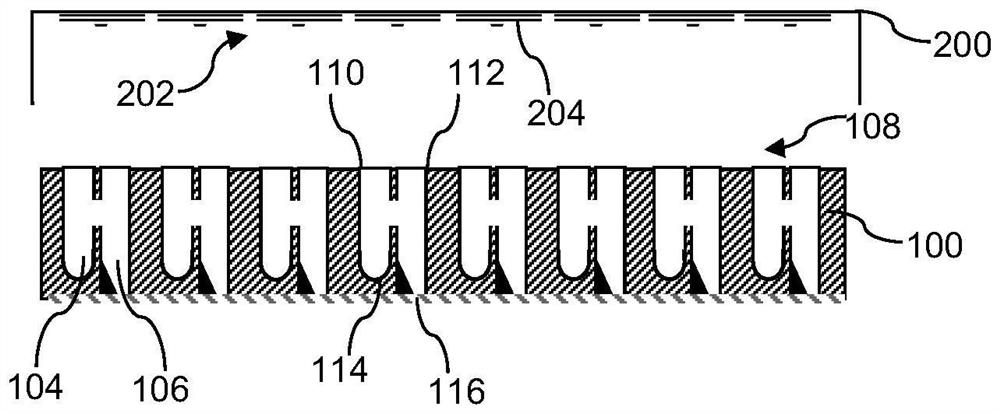
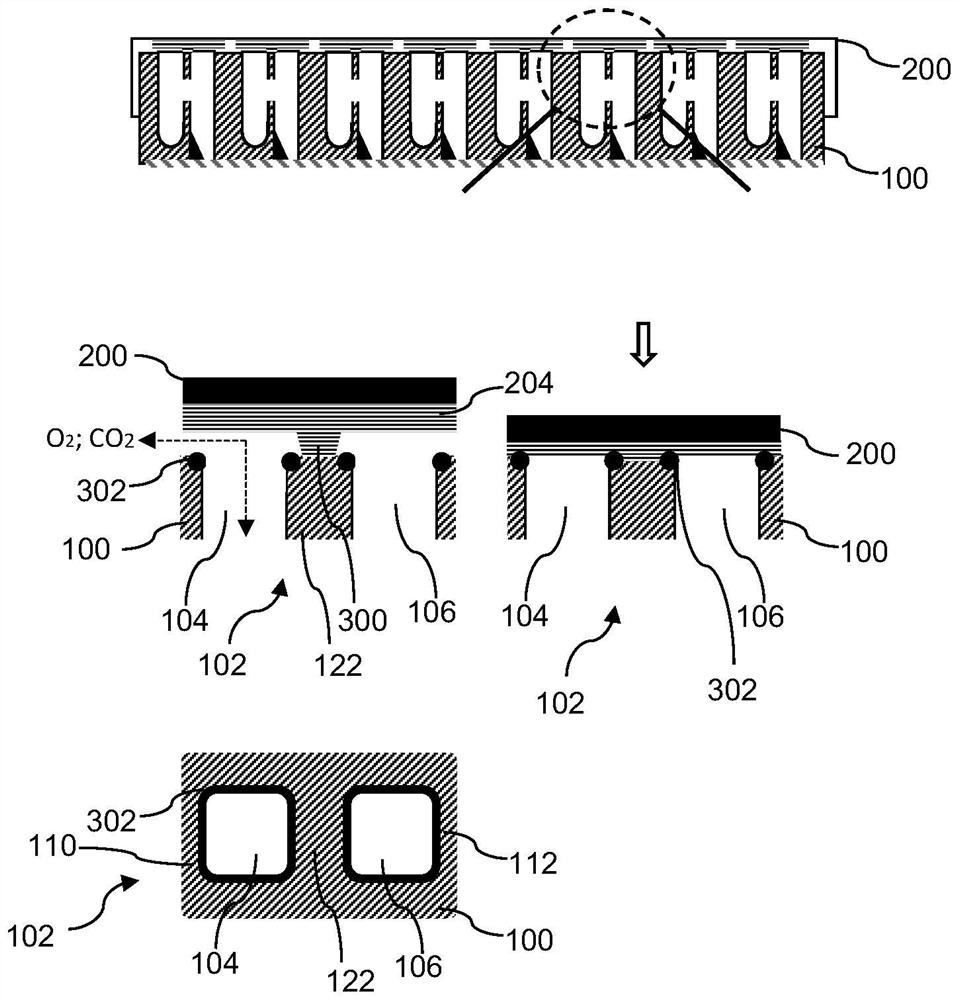
[0032] Each of the compartments 104 , 106 is open towards the top side 108 of the microplate 100 and includes openings, in particular the donor compartment 104 includes the donor opening 110 and the acceptor compartment 106 includes the acceptor opening 112 . The openings 110, 112 allow individual access to the interior of the respective compartments 104, 106, eg, for individually adding or removing samples using a manual pipette or using a liquid handling robot. figure 1 The openings 110, 112 of the microplate 100 are rectangular in shape. Similarly, the side ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com