Methods and devices for providing prolonged drug therapy
A technology of drug and drug layer, which is applied in the field of immediate release drug dosage forms and osmotic dosage forms, and can solve the problems of reduced therapeutic effect of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
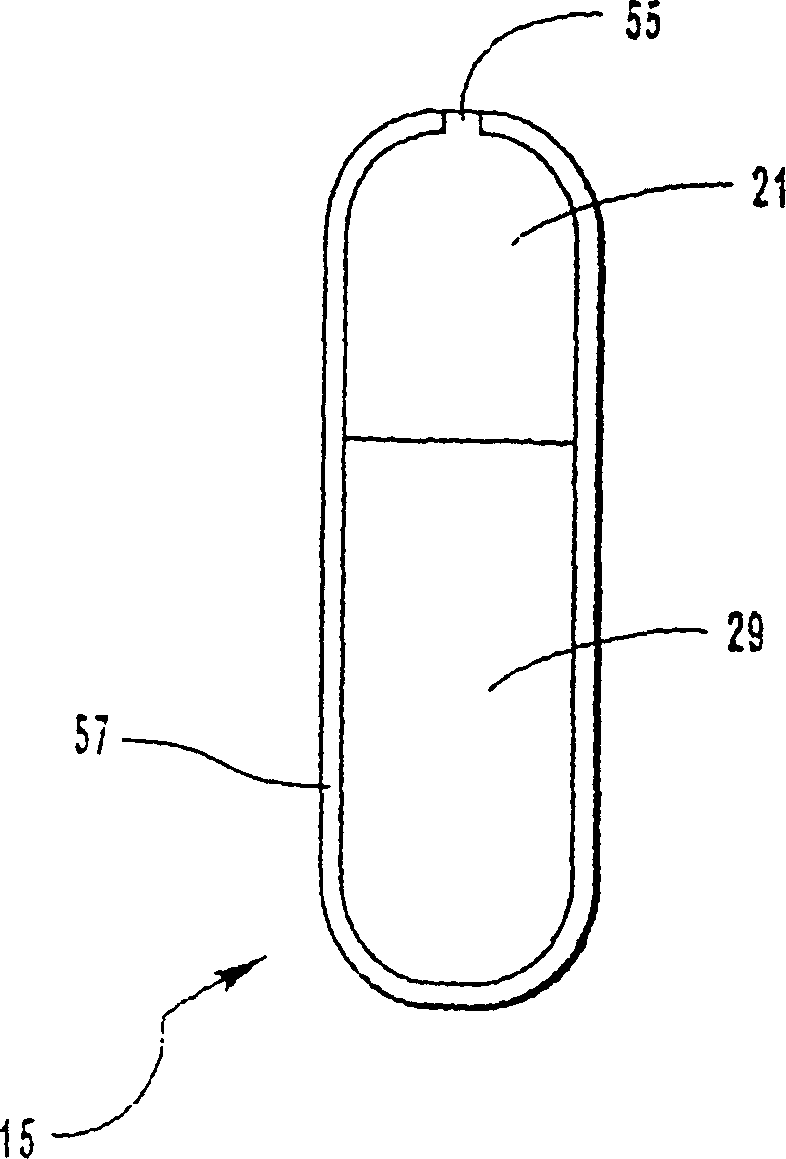
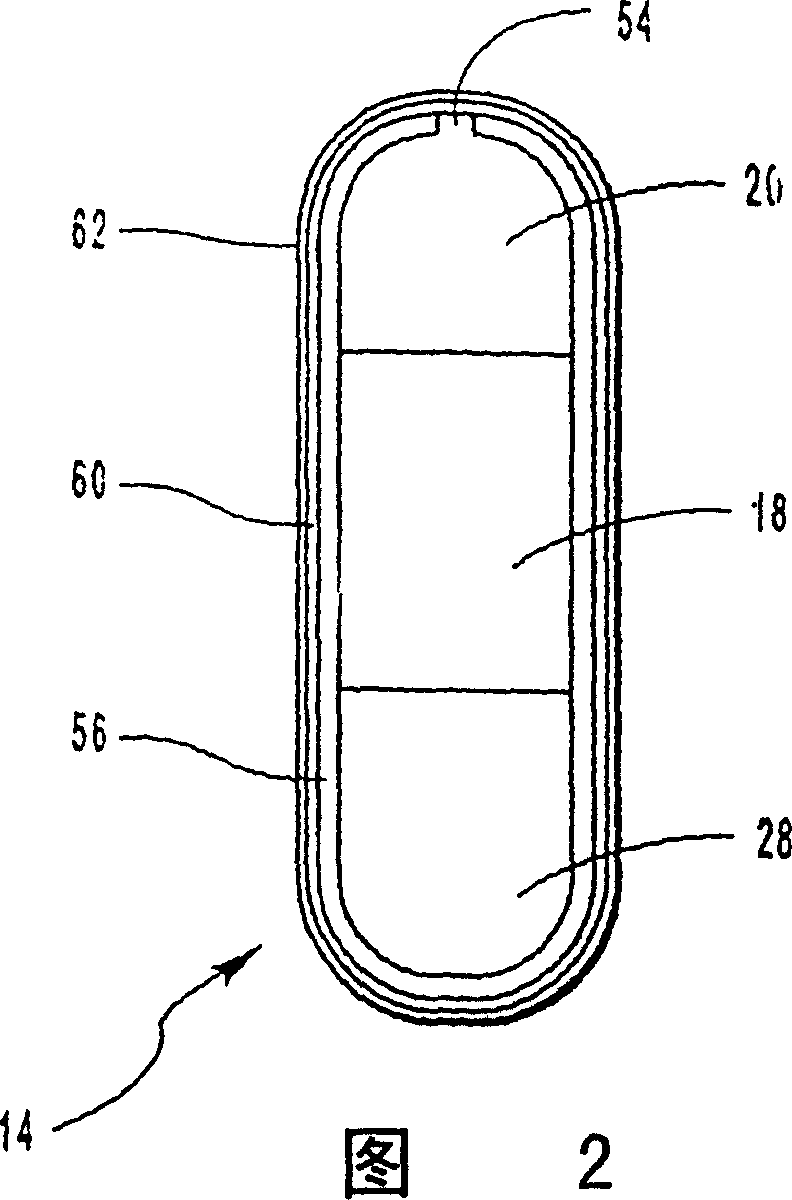
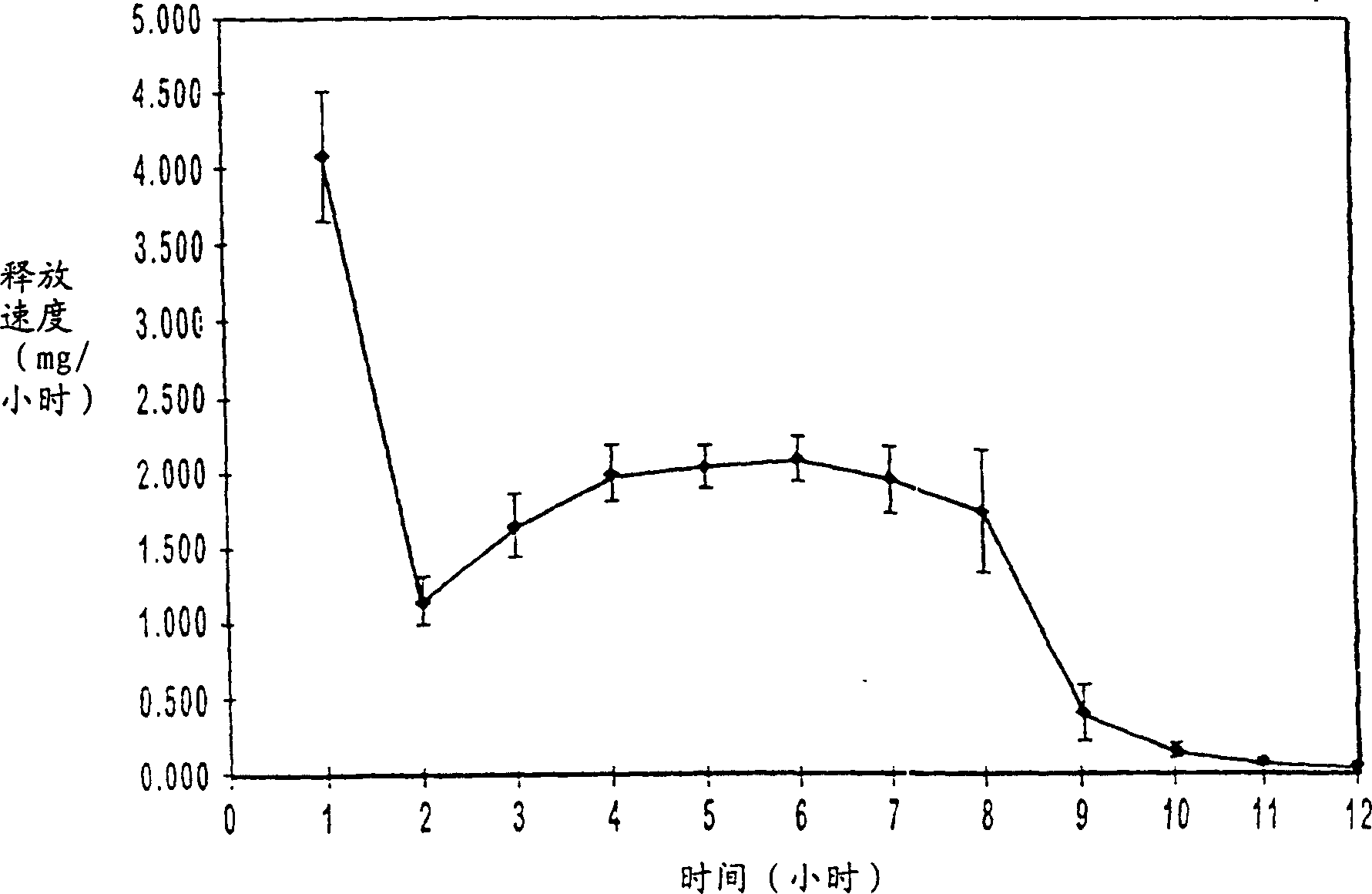
[0068] Bilayer oral osmotic dosage forms are prepared according to conventional manufacturing methods known in the art and described in detail in co-pending US Application No. 967,606, filed November 10, 1997, of which this application is a continuation-in-part. Briefly, a first component layer containing methylphenidate hydrochloride and selected excipients and a second propulsion layer containing a suitable osmopolymer, 40 wt. layer. The first component layer and the second push layer granulation formulation are then longitudinally compressed together to form a bilayer LCT core. A selected semipermeable membrane was then wrapped around the bilayer LCT core, forming suitable 30 mil pores through the membrane into the first component layer for drug release.
[0069] Each dosage form prepared contains:
[0070] first layer
[0071] 14.08mg methylphenidate hydrochloride
[0072]90.26mg polyethylene oxide (number average molecular weight 200,000)
[0073] 5.5mg polyvinylpy...
Embodiment 2
[0089] Bilayer oral osmotic dosage forms are prepared according to conventional manufacturing methods known in the art and described in detail in co-pending US Application No. 967,606, filed November 10, 1997, of which this application is a continuation-in-part. Briefly, a first component layer containing methylphenidate hydrochloride, sorbitol and selected excipients and a second layer containing a suitable osmopolymer, 40 wt. Two propulsion layers. The first component layer and the second push layer granulation formulation are then longitudinally compressed together to form a bilayer LCT core. A selected semipermeable membrane was then wrapped around the bilayer LCT core, forming suitable 30 mil pores through the membrane for drug release.
[0090] Each dosage form prepared contains:
[0091] The first ingredient layer (110mg)
[0092] 12.8% Methylphenidate Hydrochloride
[0093] 54.75% polyethylene oxide (number average molecular weight 200,000)
[0094] 25.4% Sorbit...
Embodiment 3
[0113] According to conventional preparation methods known in the art and described in detail in pending U.S. application No. 967,606 filed on November 10, 1997 (this application is a continuation-in-part thereof), a bilayer oral osmotic dosage form is prepared, which dosage form Also included is an immediate release drug dose coated on a semipermeable membrane. Briefly, a first component layer containing methylphenidate hydrochloride, sorbitol, and selected excipients and a second layer containing a suitable osmopolymer, 39.8 wt. Two propulsion layers. The first component layer and the second push layer granulation formulation are then longitudinally compressed together to form a bilayer LCT core. A selected semipermeable membrane was then wrapped around the bilayer LCT core, forming suitable 30 mil pores through the membrane for drug release. A drug-containing coat mixture is prepared and coated on the semipermeable membrane of the osmotic dosage form. Optionally, an odor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com