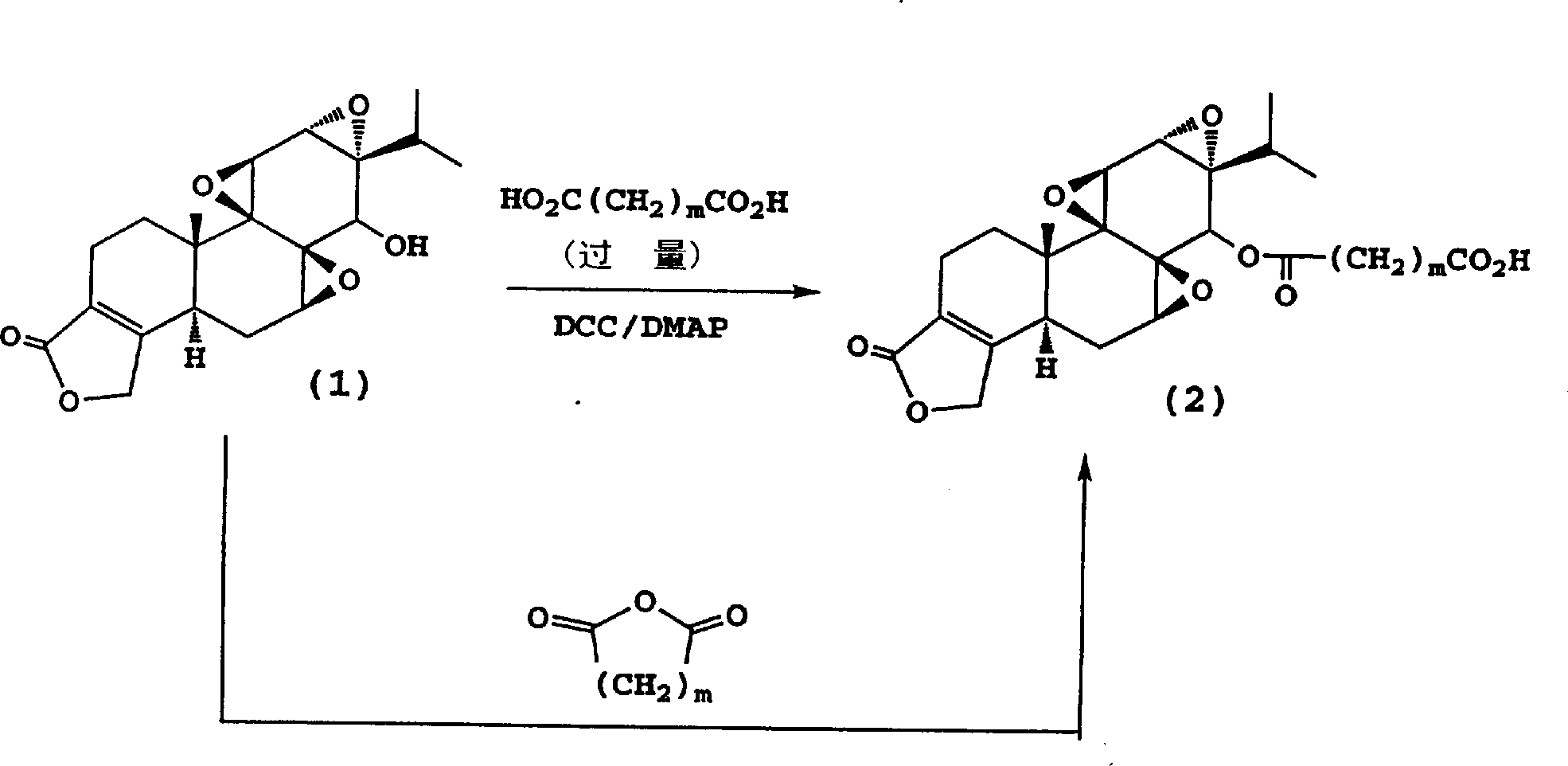
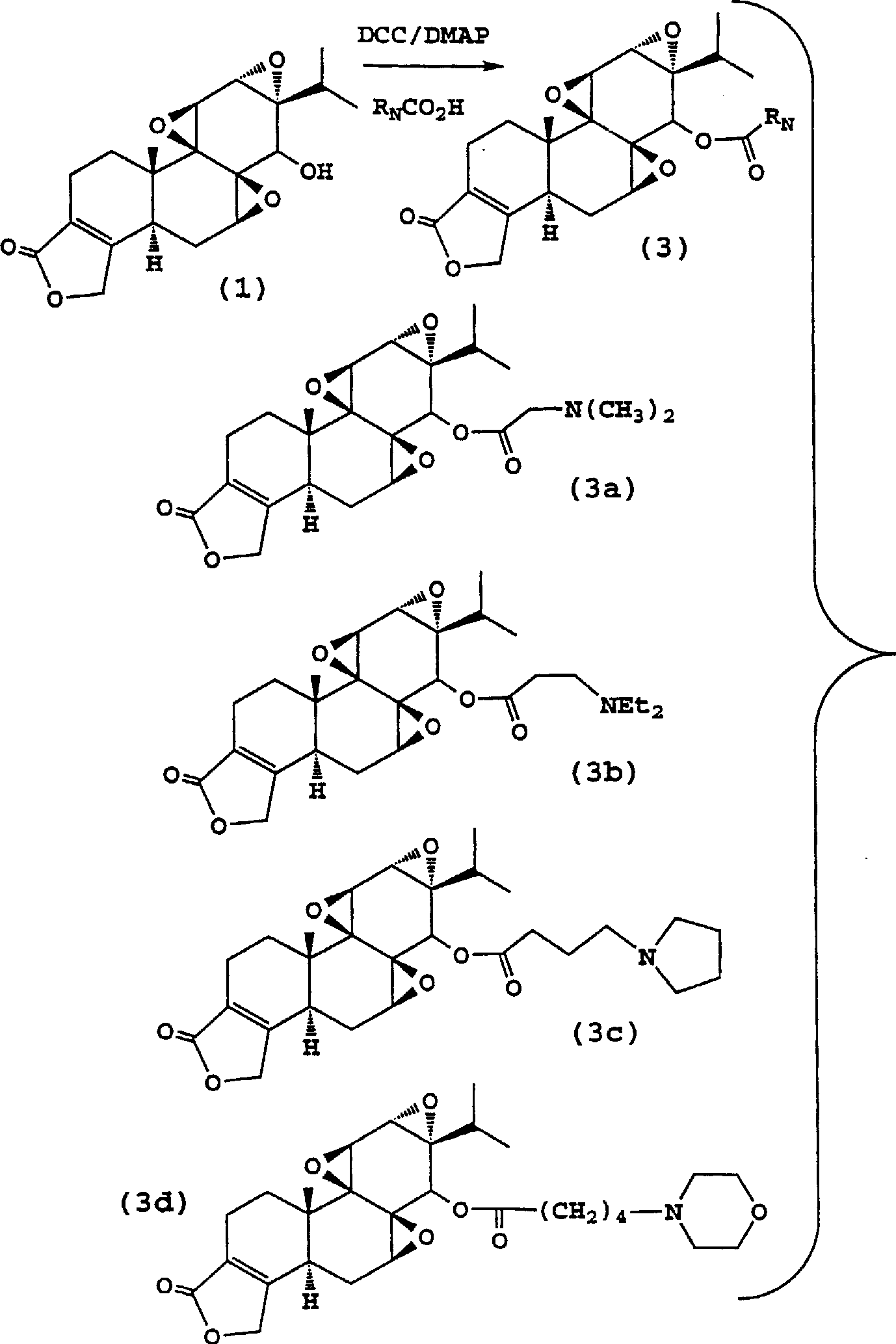
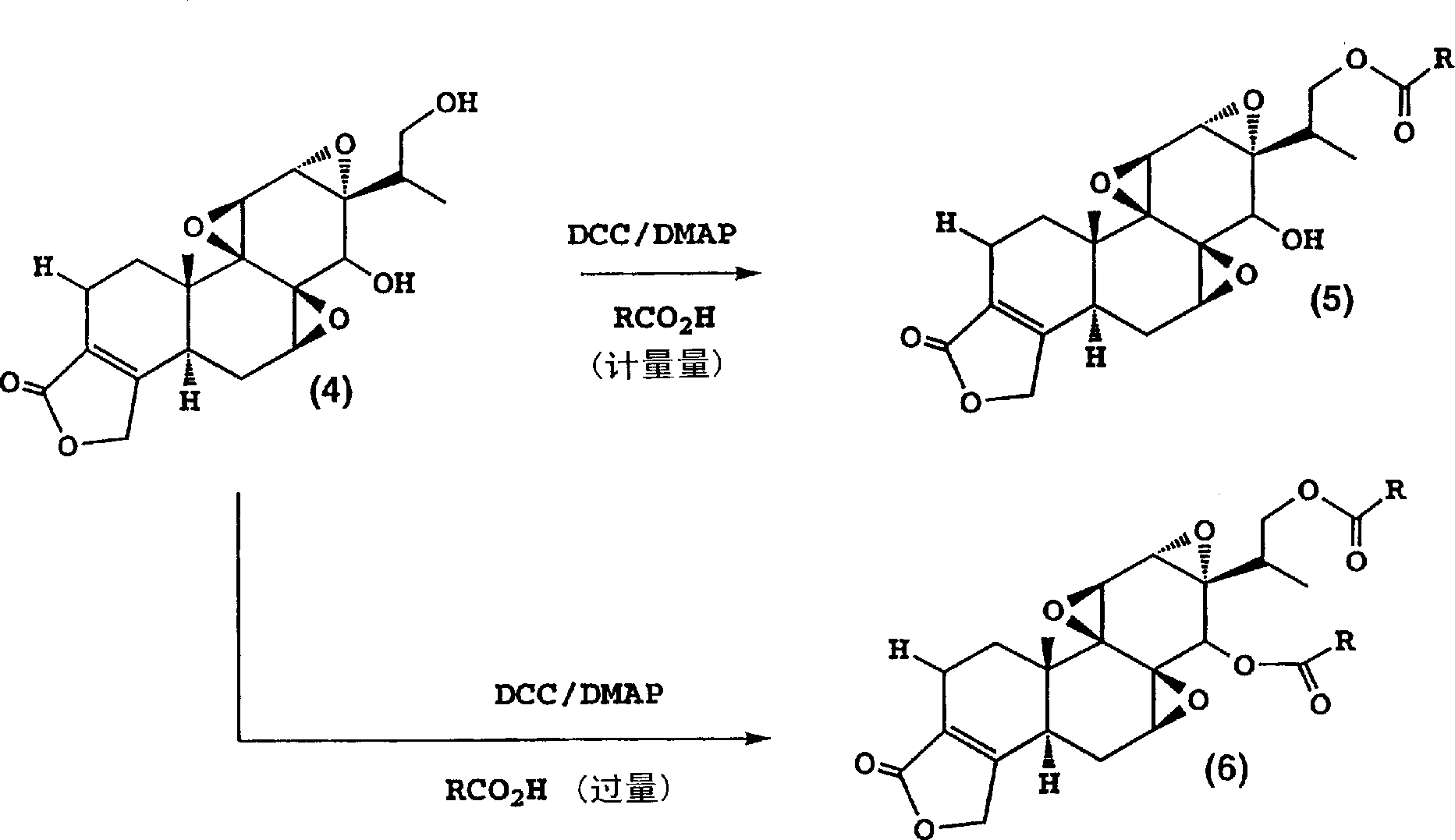
Immunosuppressive compounds and methods
A compound and pharmaceutical technology, applied in the field of immunosuppressive compounds and can solve the problems of complex suspensions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Triptolide Succinate (YM-262)
[0142] A solution of triptolide (100 mg) in 10 ml pyridine was treated with succinic anhydride (150 mg) at room temperature. The reaction was carried out at 85°C for 30 hours under nitrogen atmosphere. Hexane (50ml) was added to the resulting mixture to precipitate a crude product which was collected by filtration and washed with hexane. Recrystallization from ether / hexane gave 90 mg (70%) of triptolide succinate (YM-262), m.p.: 111-113°C.
[0143] IR(KBr): 3431.8, 2974.6, 1375.5, 1743.8, 1159.4, 1022.4 cm -1 . 1 HNMR (CDCl 3 ): 5.08 (1H, s, 14-CH), 4.67 (2H, s, 19-CH2), 3.82 (1H, d, 11-CH), 3.50 (1H, d, 12-CH), 3.43 (1H, d, 7-CH), 2.75 (5H, m, CH2CH2, 5-CH), 2.30 (1H, d-m, 15-CH), 2.15 (2H, m, 6-CH a , 2-CH a ), 1.88 (2H, m, 2-CH b ,6-CH b B), 1.55 (1H, m, 1-CH b ), 1.20 (1H, m, 1-CH 2 ), 1.05(3H, s, 20-CH3), 0.95(3H, d, 16-CH 3 ), 0.83 (3H, d, 17-CH 3 )ppm. MS (m / z): 461 (M+1).
Embodiment 2
[0145] Triptolide succinate trisalt (YM-273)
[0146] A solution of triptolide succinate (20 mg) and tris(hydroxymethyl)aminomethane (5.3 mg) in 20 ml of water was mixed and stirred for 1 hour. The solution was filtered and the filtrate was lyophilized to give 24 mg (96%) of a white powder.
[0147] IR(KBr): 3391, 2937.96, 1745.80, 1562.9, 1411.67, 1159, 1066.57, 1024.57cm -1 . 1 H NMR (D 6 -DMSO, ppm): 5.00 (1H, s, 14-CH), 4.85 (2H, d, 19-CH2), 3.95 (1H, d, 11-CH), 3.70 (1H, d, 12-CH), 3.55 (1H, d, 7-CH), 3.30 (6H, s, 3CH 2 O), 2.65 (1H, m, 5H), 2.45 (2H, m, CH2), 2.20 (3H, m, CH 2 , 15-CH), 1.90 (4H, m, 6-CH 2 , 2-CH2), 1.34 (2H, br, 1-CH2), 0.95 (3H, s, 20-CH3), 0.88 (3H, d, 16-CH3), 0.75 (3H, d, 17-CH 3 ).
Embodiment 3
[0149] Triptolide Sodium Succinate (YM-274)
[0150] A solution of triptolide succinate (20 mg) and sodium bicarbonate (3.65 mg) in 20 ml of water was mixed and stirred for 30 minutes. The aqueous solution was filtered and the filtrate was lyophilized to give 20 mg (95%) of a white powder.
[0151] IR(KBr): 3431.8, 2975.56, 1743.87, 1577.97, 1419.79, 1163.22, 1022.40cm -1 . 1 H NMR (D 6 -DMSO, ppm): 5.00 (1H, s, 14-CH), 4.85 (2H, d, 19-CH2), 3.95 (1H, d, 11-CH), 3.70 (1H, d, 12-CH), 3.55 (1H, d, 7-CH), 2.58 (1H, m, 5H), 2.45 (2H, m, CH2), 2.20 (3H, m, CH2, 15-CH), 1.90 (4H, m, 6- CH2, 2-CH2), 1.34 (2H, br, 1-CH2), 0.95 (3H, s, 20-CH3), 0.88 (3H, d, 16-CH3), 0.75 (3H, d, 17-CH3) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com