Tripterygium wilfordii lactone prodrugs having high aqueous solubility
A technology of inorganic esters and polar groups, applied in the field of triptolide prodrugs with high water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
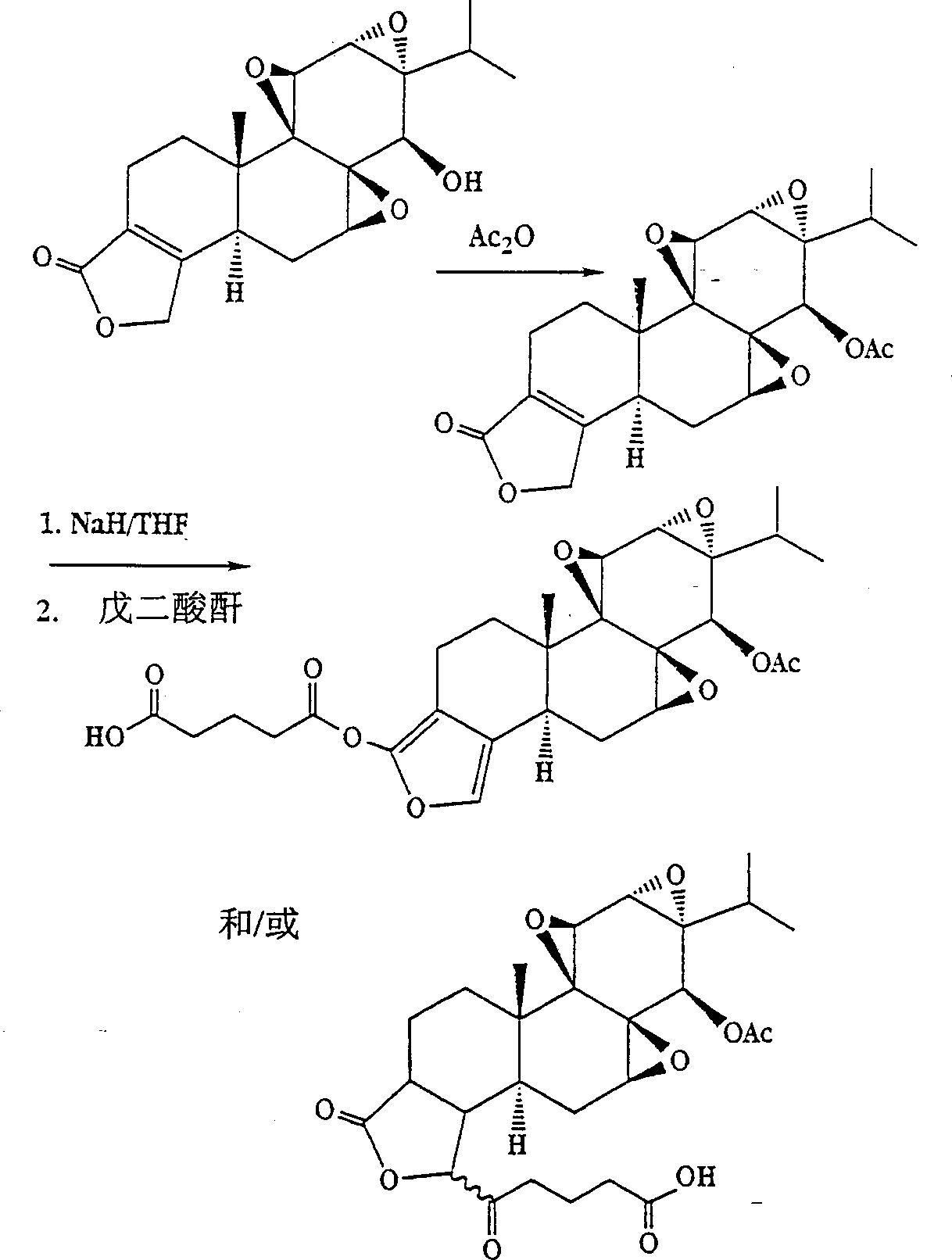
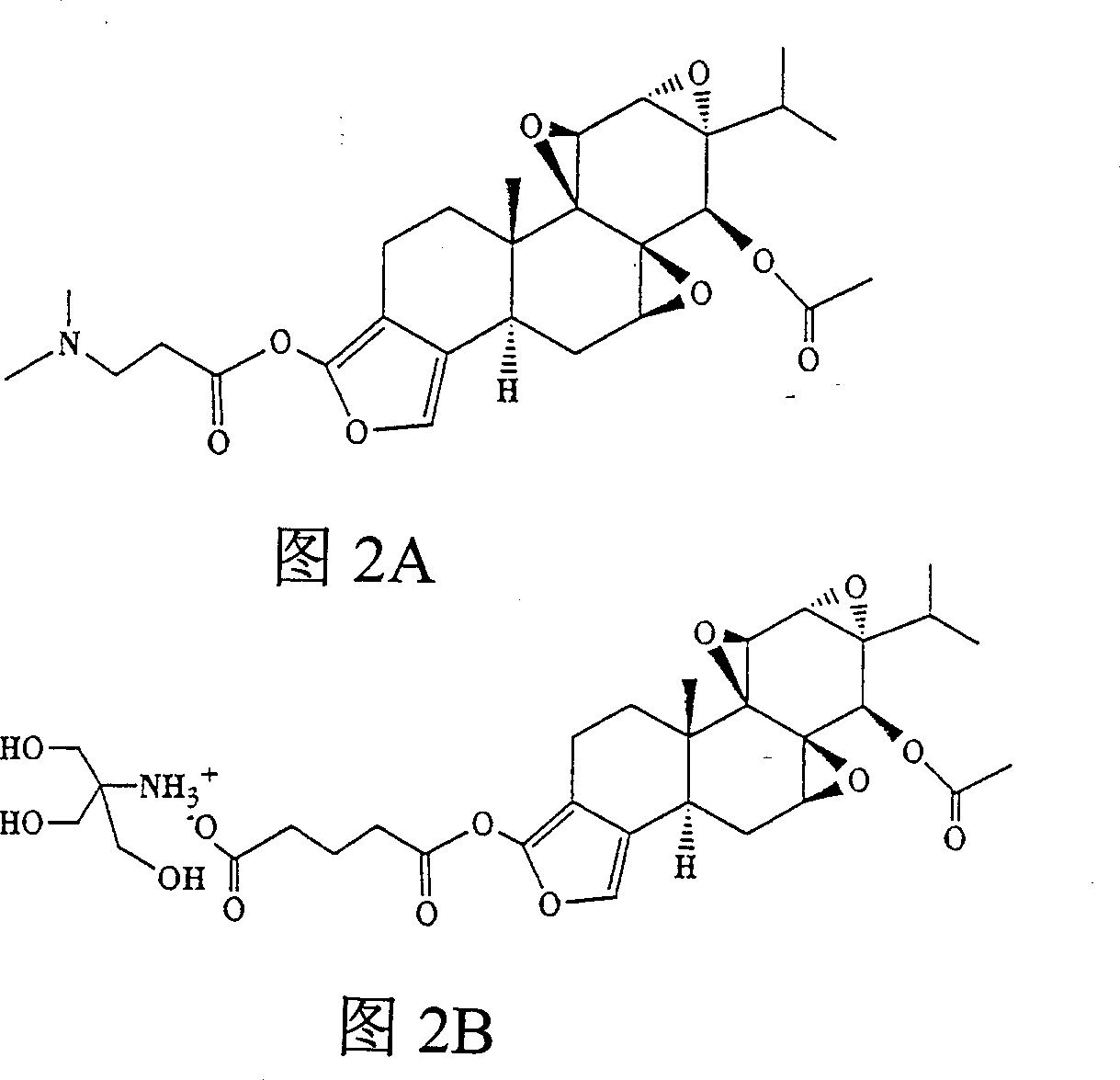
[0059] Example 1 Synthesis of 18-glutarylfuranoid triptolide analogs ( figure 1 ) and tromethamine salt (Figure 2B)
[0060] Triptolide (1 equiv) in anhydrous THF was added dropwise to a slight excess of NaH suspension in anhydrous THF at -78°C under an inert atmosphere. After about 0.5 hour, glutaraldehyde (1 equivalent or a slight excess) was added dropwise, and the mixture was stirred and allowed to come to room temperature over about 1 hour. The mixture was concentrated, dissolved in ether, washed with water and brine, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by chromatography on silica gel.
[0061] To a stirred solution of 1 equivalent of glutaryl ester in THF was added a slight excess of tromethamine in methanol. The solution was concentrated, the salt was recovered and dried in vacuo.
Embodiment 2
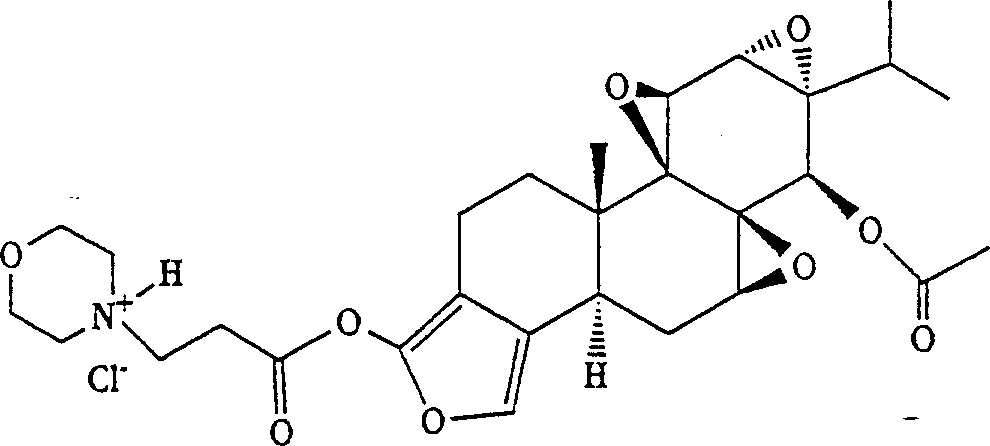
[0063] Synthesis of triptolide analogs with lactone ring opening ( image 3 )
[0064] Triptolide, excess (2-hydroxyethyl)morpholine, and catalytic amount of DMAP (dimethylaminopyridine) are stirred in a polar aprotic solvent such as THF with moderate heating. The reaction was monitored by TLC. Once the reaction is complete, the reaction mixture is cooled and acetic anhydride is added to acetylate the 14- and 19-hydroxyl groups. The solvent was removed under reduced pressure, the residue was dissolved in ether, washed several times with water and sodium bicarbonate, dried over anhydrous magnesium sulfate and concentrated. The product was purified by silica gel chromatography as required.
Embodiment 3
[0066] Synthesis of 12-tosyloxy-13-hydroxy triptolide ( Figure 5 )
[0067] The 14-hydroxyl group was first protected by converting it to a benzyl ether. In order to avoid the reaction of the acidic hydrogen of the conjugated lactone with basic reagents such as metal hydrides, this compound (1 equivalent) was mixed with BzBr (2.5 equivalents) on Ag 2 Reaction in DMF in the presence of O (2 equiv.) (see eg Mori et al.). The mixture was brought to room temperature with stirring and stirred for about 24 hours. The mixture was diluted with ether, washed with water and brine, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by chromatography on silica gel, if necessary.
[0068] The resulting 14-O-benzyltriptolide was then heated with sodium hydroxide in aqueous THF to convert the 12,13-epoxide into a diol. The solution was concentrated and the residue was dissolved in ether and worked up as above.
[0069] The resulting diol (1 eq) was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com