Heterofluorene derivative with strong two photon absorption character
A technology of two-photon absorption and derivatives, applied in organic chemistry and other fields, can solve the problems of high price and difficult synthesis of thiophene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
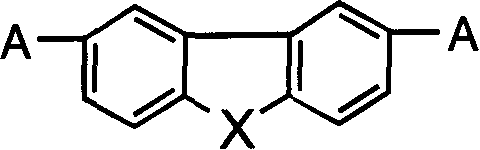
[0021] Example 1: 2,7-bis[(2-4'-ethoxyphenyl-5-4'-styryl)-1,3,4-oxadiazole] thiafluorene and its synthesis.
[0022] (1) Add 40.0g (0.206mol) of homemade ethyl 4-ethoxybenzoate, 30ml of hydrazine hydrate (30.9g, 0.617mol), and 200ml of absolute ethanol into a single-neck round-bottomed flask, and reflux for 1~ 4 days. The solvent was distilled off and filtered with suction to obtain 20 g of white solid 4-ethoxy benzoyl hydrazide (a), mp: 125-128°C.
[0023] (2) Mix compound (a), pyridine, and tetrahydrofuran in a beaker. Under stirring, the mixed solution was slowly added to 15.4g of 4-methylbenzoyl chloride, and a white precipitate was immediately formed. After stirring for several hours at room temperature, it was slowly poured into ice water, allowed to stand overnight, filtered, and recrystallized with absolute ethanol to obtain 8.3 g of bishydrazide intermediate (b).
[0024] (3) Add POCl sequentially to the round bottom flask 3 And intermediate (b), N 2 Reflux for 4-8 hours ...
Embodiment 2 to Embodiment 21
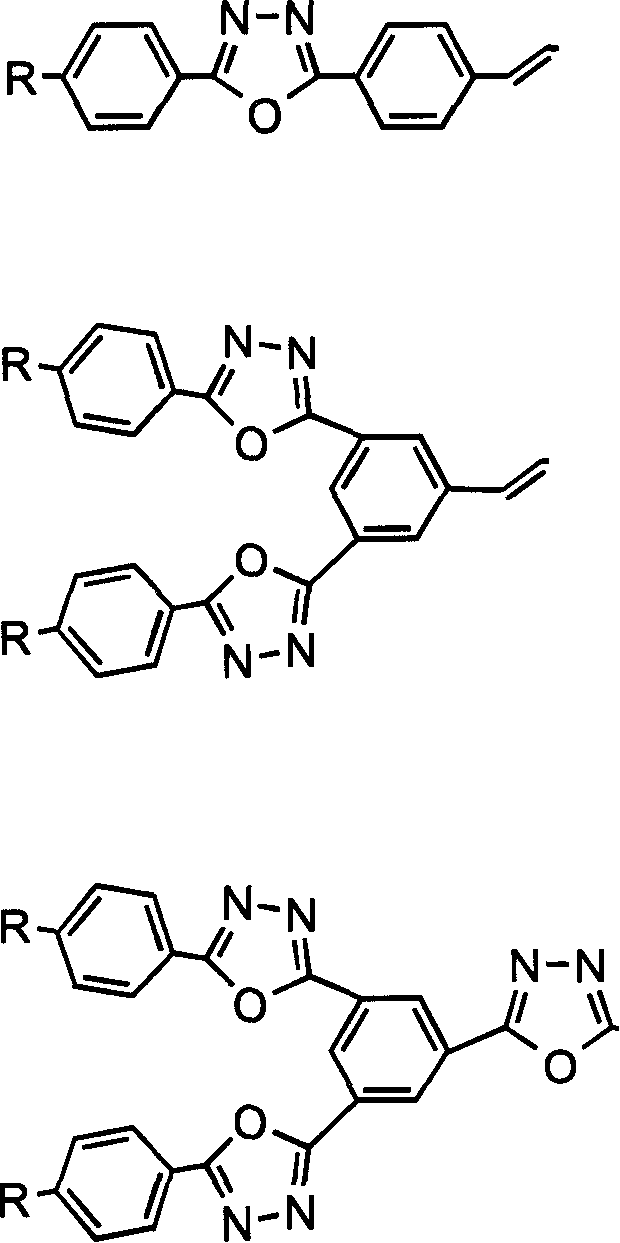
[0032]Example 2 to Example 21: The synthesis method is similar to that of Example 1. It only needs to change the ethyl 4-ethoxybenzoate in step (1) and the 2,7- in step (6) according to the obtained compound. Thiofluorene dialdehyde.
[0033] The general formula of the compound is,
[0034]
[0035] Example
R
X
Example 2
C(CH 3 ) 3
S
Example 3
F
S
Example 4
OC 2 H 5
O
Example 5
C(CH 3 ) 3
O
Example 6
F
O
Example 7
OC 2 H 5
NC 2 H 5
Example 8
OC 2 H 5
NC 4 H 9
Example 9
OC 2 H 5
NC 8 H 17
Example 10
OC 2 H 5
NC 12 H 25
Example 11
OC 2 H 5
NC 22 H 45
Example 12
C(CH 3 ) 3
NC 2 H 5
Example 13
C(CH 3 ) 3
NC 4 H 9
Example 14
C(CH 3 ) 3
NC 8 H 17
Example 15
C(CH 3 ) 3
NC 12 H 25
Example 16
C(...
Embodiment 22
[0039] Example 22: Prepared according to the following steps,
[0040] (1) Dissolve 1-cyano-3,5-phthalic acid in 50ml SOCl 2 In the medium, 10ml of pyridine was added dropwise and refluxed at 55°C for 7 hours to obtain a dark brown transparent liquid. Excess SOCl 2 Distilled with pyridine under reduced pressure to obtain 1,3-diacyl chloride benzocyanide as a yellow solid.
[0041] (2) Dissolve 9.36g (0.052mol) of 4-ethoxybenzoic acid hydrazide in the mixture of THF and pyridine, and then add dropwise to 1,3-diacyl chloride benzonitrile, and stir at room temperature for 4-6 hours. The THF was distilled off under reduced pressure, the remaining liquid was poured into water, and a white solid precipitated out after standing for 2 hours, filtered with suction and washed with ethanol to obtain 8 g of intermediate product (I) with a yield of 66%. M.p.252-255°C. Dissolve 10g (0.021mol) of intermediate product (I) in 125ml POCl 3 Yes, the reaction solution is in N 2 Reflux under the envir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com