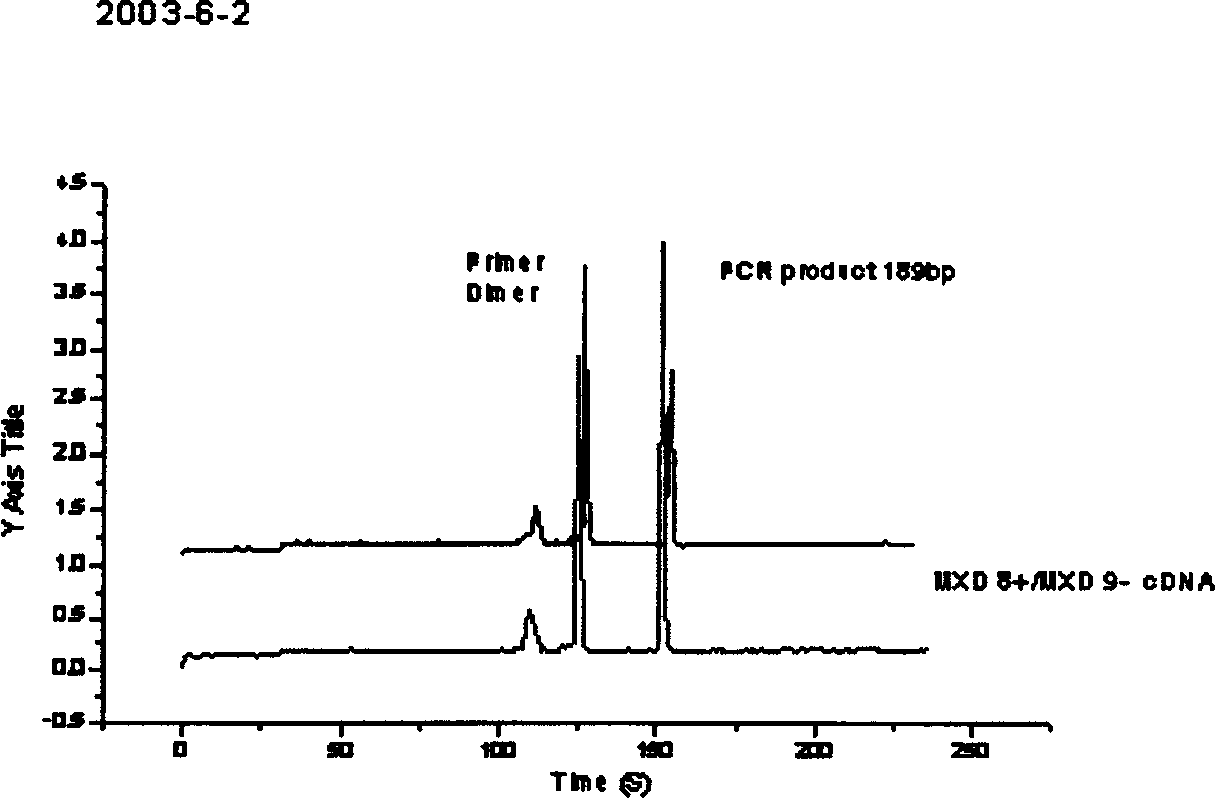
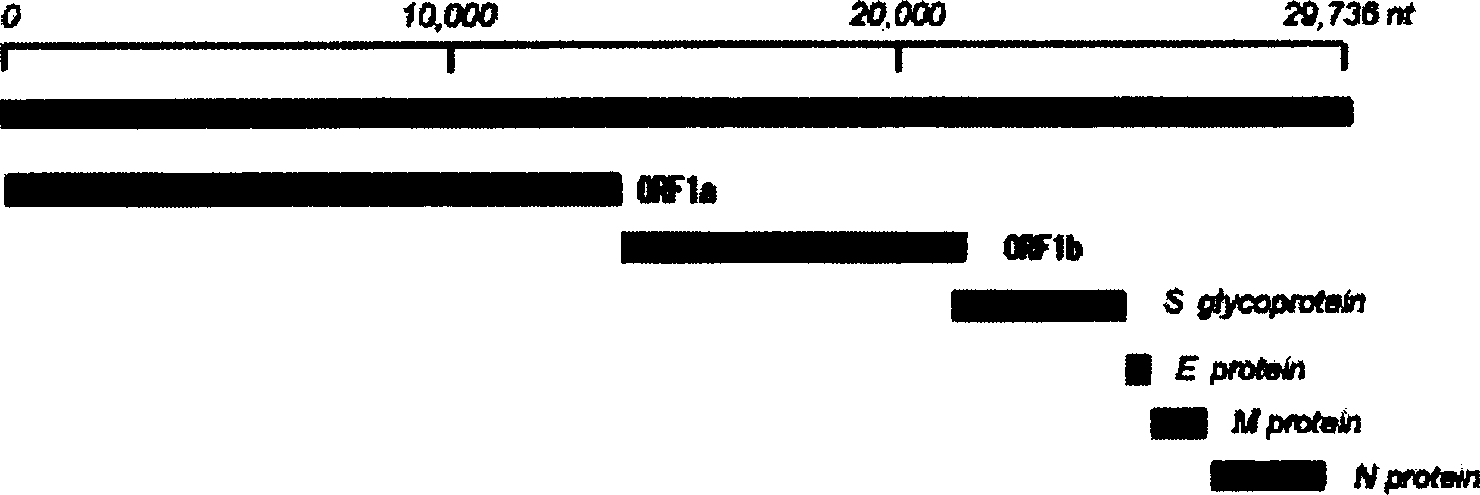Diagnosis reagent box for detecting SARS coronavirus using transcription PCR method
A diagnostic kit, coronavirus technology, applied in biochemical equipment and methods, microbiological determination/inspection, resistance to vector-borne diseases, etc., can solve problems such as no comparability, no publication of clinical statistics, and different sequences , to achieve the effect of improving sensitivity, high sensitivity, and overcoming false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1. Kit 1
[0103] 1) Materials: Positive specimens are from the National Academy of Military Medical Sciences, and the samples are genetically engineered Vero-6 virus cell strains with SARS virus RNA; stored at -80C for later use. Control substances were normal human saliva and other viruses.
[0104] 2) Preparation of RNA sample: the preparation of RNA sample is referring to NEJM (document 13.Marco A.Marra, Steven J.M.Jones, Caroline R.Astell etc, The Genome Sequenceof the SARS-Associated Coronavirus, Science, www.scienceexpress.org1May 2003) , that is, add an equal volume of N-acetyl cysteine buffer solution (0.9% NaCl fusion solution, concentration of 10g / l N-acetyl cysteine buffer solution) to the body fluid containing SARS cells; shake slowly 30 minutes; take 600 μL of this solution, centrifuge (10,000 g) in a conventional desktop centrifuge for 3 minutes, add 140 μL of supernatant to 560 μL of AVL buffer (Qiagen viral RNA kit), redissolve the cell pel...
Embodiment 2
[0218] Embodiment 2. Kit 2
[0219] Primers used primer pair BIT001, and other parts of the kit were the same as Example 1.
Embodiment 3
[0220] Embodiment 3. Kit 3
[0221] Primers used primer pair BIT002, and other parts of the kit were the same as Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com