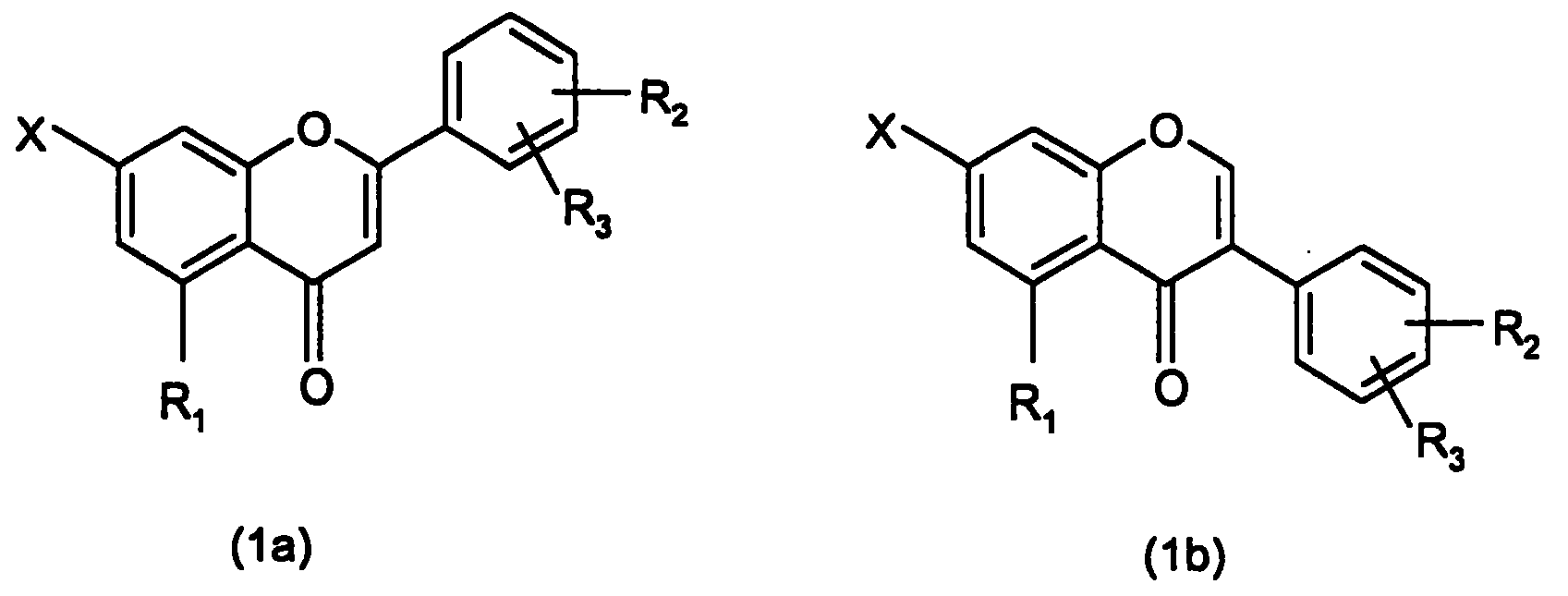
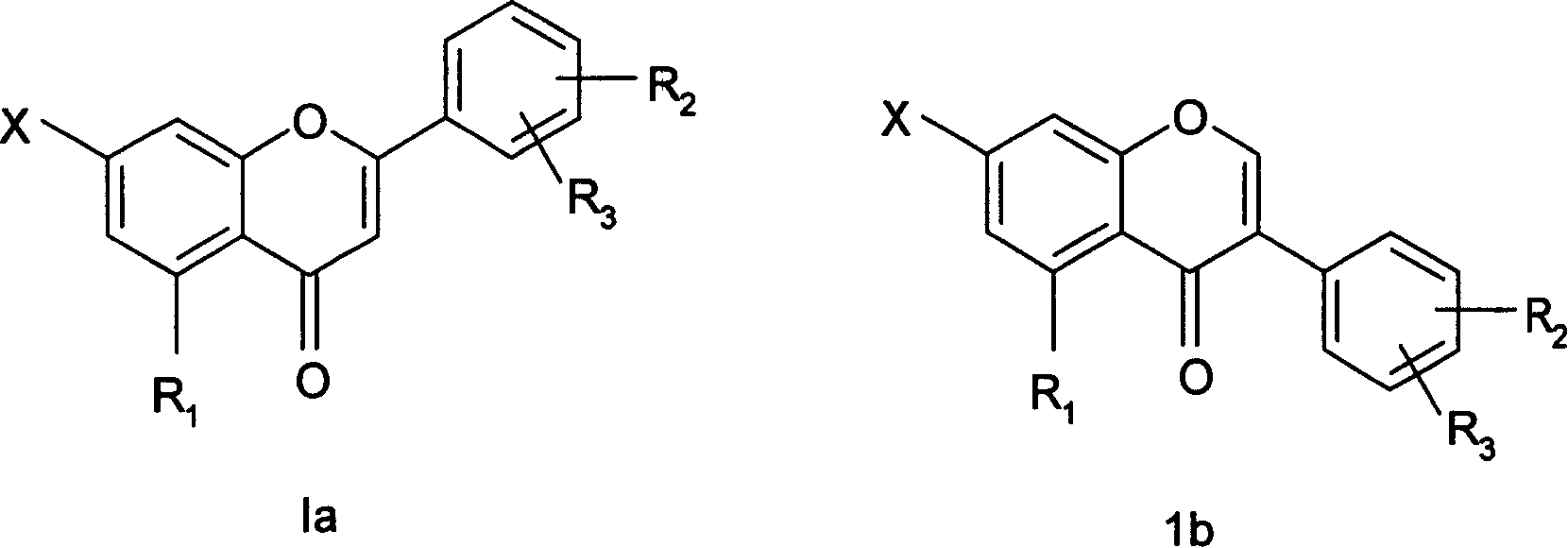
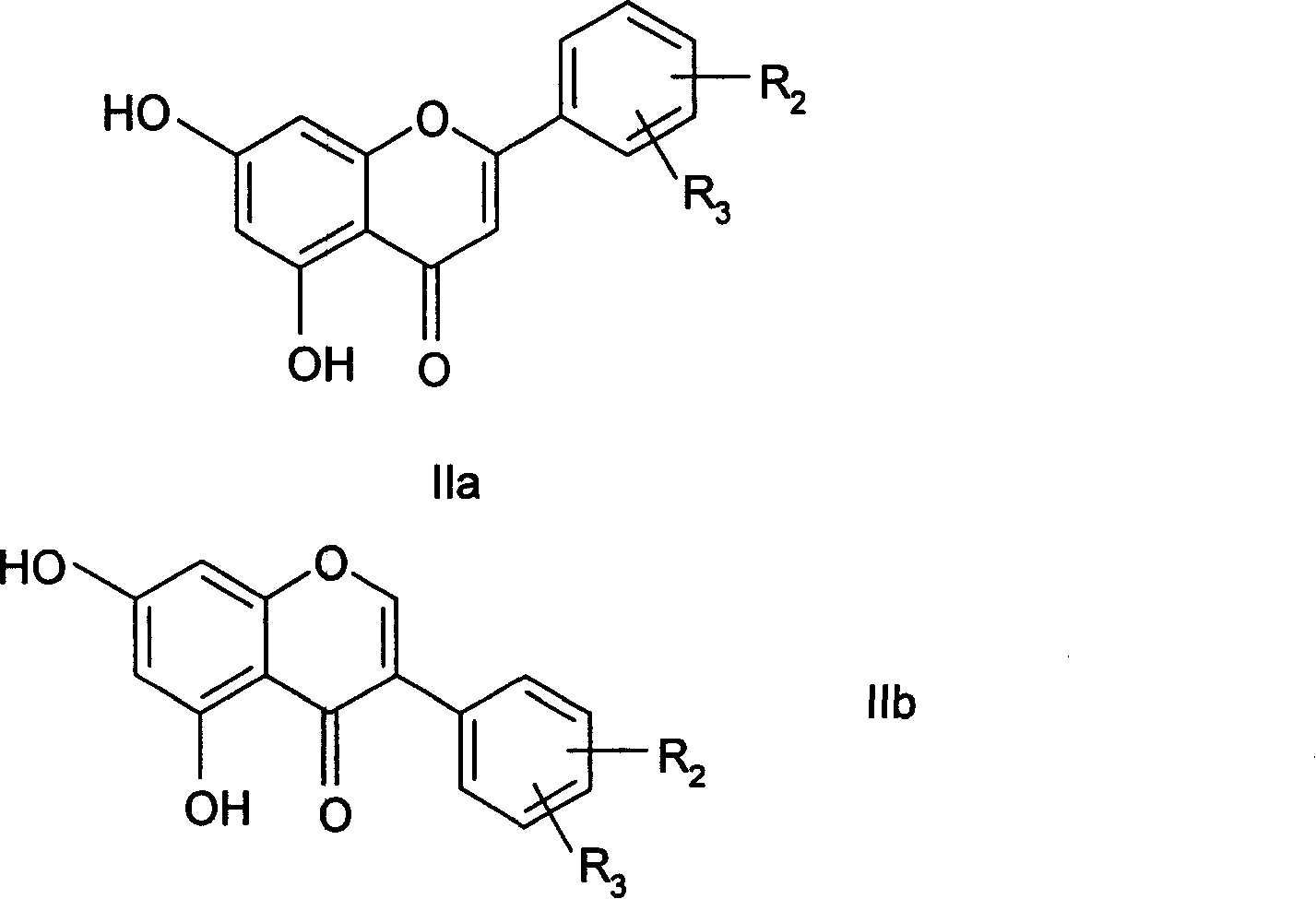
7-carboxy-flavone derivatives, preparation method and therapeutic use
A kind of derivative, the technology of dihydroxyflavonoids, applied in the field of novel flavonoids and isoflavone derivatives, can solve problems such as serious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 5-Hydroxy-2-phenyl-4-oxo-4H-chromene-7-carboxylic acid
[0055] 12.7 ml of pyridine and 6.6 ml of trifluoromethanesulfonic anhydride were successively added to 200 ml of a dichloromethane solution containing 10 g of 5,7-dihydroxyflavone while maintaining the temperature at 0°C. After reacting at 0°C for about 3 hours, the reaction mixture was neutralized with 1N hydrochloric acid solution, followed by extraction with dichloromethane.
[0056] After evaporation of the solvent, 13.9 g (92% yield) of 7-trifluoromethanesulfonyloxy-5-hydroxy-2-phenyl-4-oxo-4H-chromene was obtained as a white powder, the chemical structure of which was given by Confirmed by chromatography and infrared spectroscopy and calibrated by NMR and mass spectroscopy.
[0057] Rf=0.58 (AcOEt / PE=30 / 70)
[0058] IR (cm -1 ): 1655 (C=O), 1620 (C=C), 1436 (S=O)
[0059] To a solution of the above-obtained product (330 mg) in 4 ml of pyridine was added 0.16 ml of pivaloyl chloride at 0°C, an...
Embodiment 2
[0073] 7-Dimethoxyphosphoryl-5-hydroxy-2-phenyl-4-oxo-4H-chromene
[0074] The intermediate product 7-trifluoromethanesulfonyloxy-2-phenyl-5-pivaloyloxy-4-oxo-4H-chromene (500 mg) obtained in Example 1 was treated with 0.12 ml of phosphorous acid Dimethyl ester treatment in which 0.24 ml of diisopropylethylamine and 61 mg of tetrakis(triphenylphosphine)palladium in 2.5 ml of acetonitrile were present.
[0075] After heating at 70°C for 7 hours, the reaction mixture was neutralized with 1N hydrochloric acid solution, followed by extraction with dichloromethane.
[0076] After separation by flash chromatography (eluent: AcOEt / PE=5 / 95-50 / 50), 383 mg of 7-dimethoxyphosphoryl-2-phenyl-5-pivaloyloxy was obtained as a white powder - 4-Oxo-4H-chromene (84% yield), melting point Mp = 165-167°C.
[0077] The above derivative was added to sodium hydroxide solution (5ml), water (10ml) and methanol (10ml). After reacting at room temperature for 23 hours, the reaction mixture wa...
Embodiment 3
[0081] 7-Dihydroxyphosphoryl-5-hydroxy-2-phenyl-4-oxo-4H-chromene
[0082] 0.55 ml of bromotrimethylsilane were added to a solution of the product obtained in Example 2 in 13 ml of dichloromethane. After reacting at room temperature for 21 hours, the solvent was evaporated.
[0083]After recrystallization from a methanol / chloroform mixture, 7-dihydroxyphosphoryl-5-hydroxy-2-phenyl-4-oxo-4H-chromene was obtained as a yellow powder in a yield of 68%.
[0084] Melting point Mp=293-296°C (decomposition)
[0085] IR (cm -1 ): 1655 (C=O), 1616 (C=C), 1261 (P=O)
[0086] 1 H NMR (DMSO) δppm: 6.98 & 7.44 (2s, 2H, J=13.5Hz, H-6 & H-8), 7.16 (s, 1H, H-3), 7.59-7.65 (m, 3H, H- 3'&H-4'), 8.15(d, 2H, J=6.9Hz, H-2'), 12.63(s, OH)
[0087] 13 C NMR (DMSO) δppm: 107.7 (C-3), 110.8 (C-4a), 111.3 & 113.9 (J=9Hz, C-6 & C-8), 128.4 (C-2′), 130.9 (C- 3'), 132.1(C-1'), 134.2(C-4'), 156.9 & 161.1(J=20Hz, C-5, C-2, C-8a), 166.3(C-2), 183.2( C-4)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com