Acetyl salicyl puerarin derivatives and preparation method and use thereof
A technology of acetylsalicylic acid and acetylsalicylic acid chloride is applied in the field of medicine to achieve the effects of improving cell membrane permeability, improving oral bioavailability and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
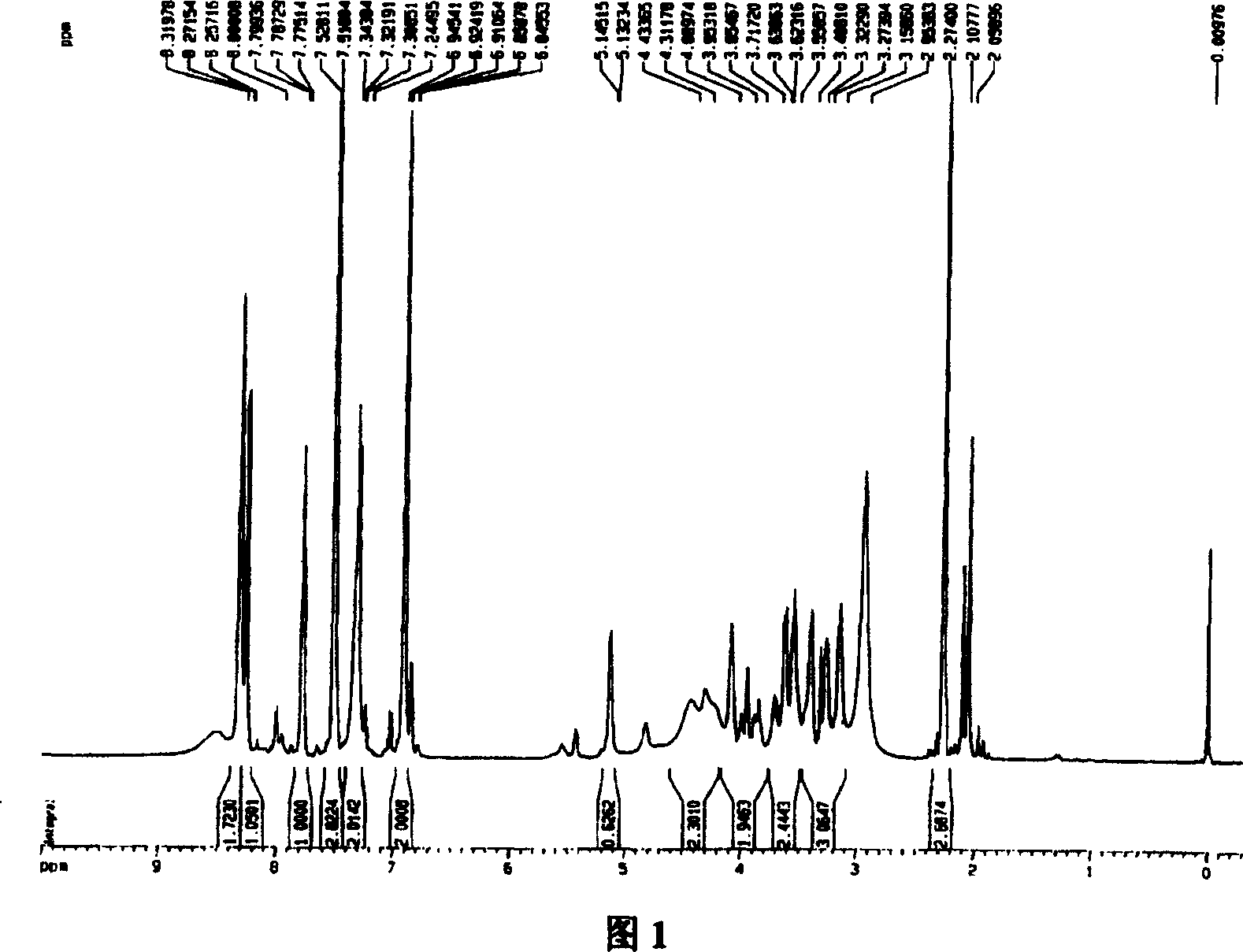
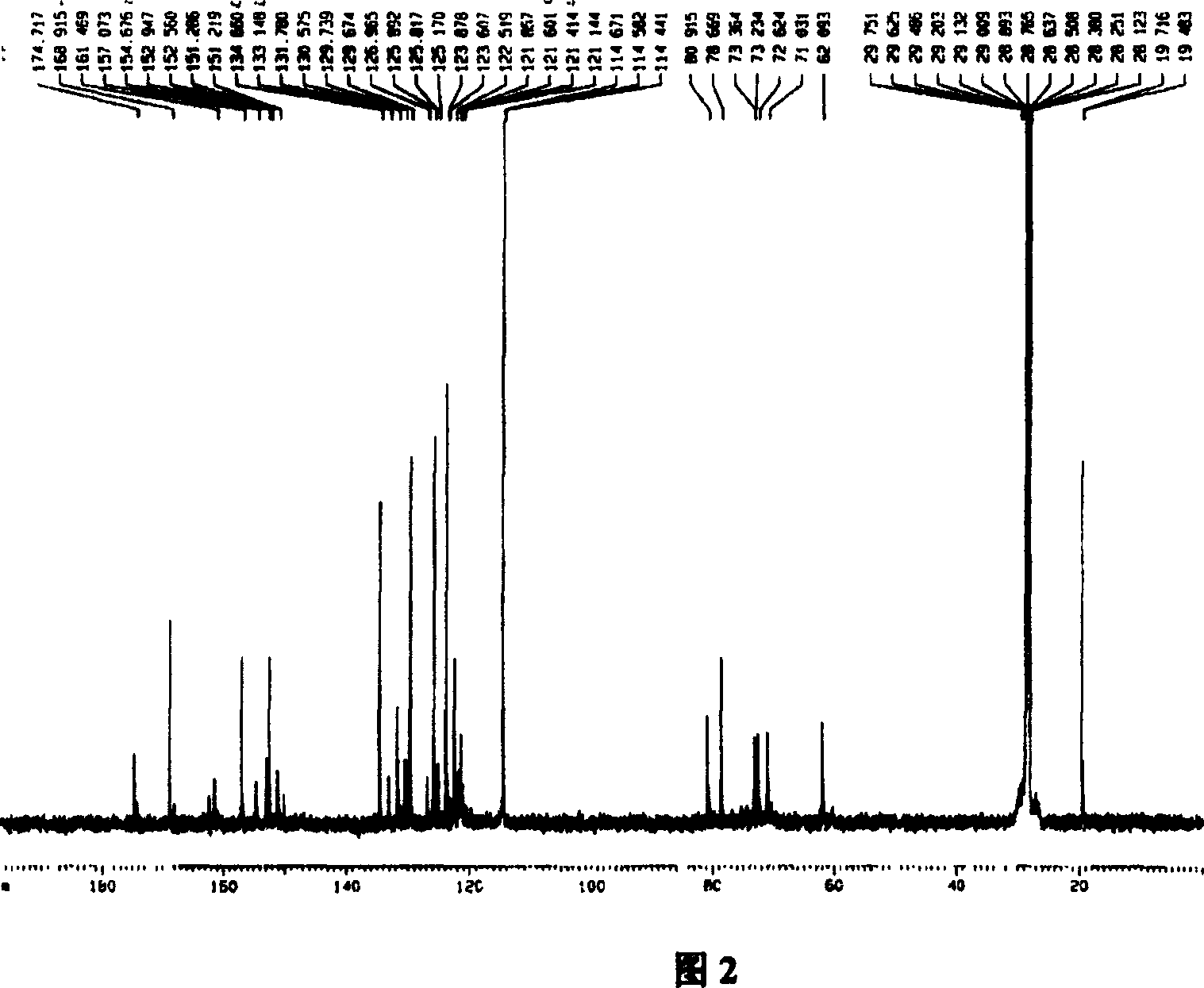
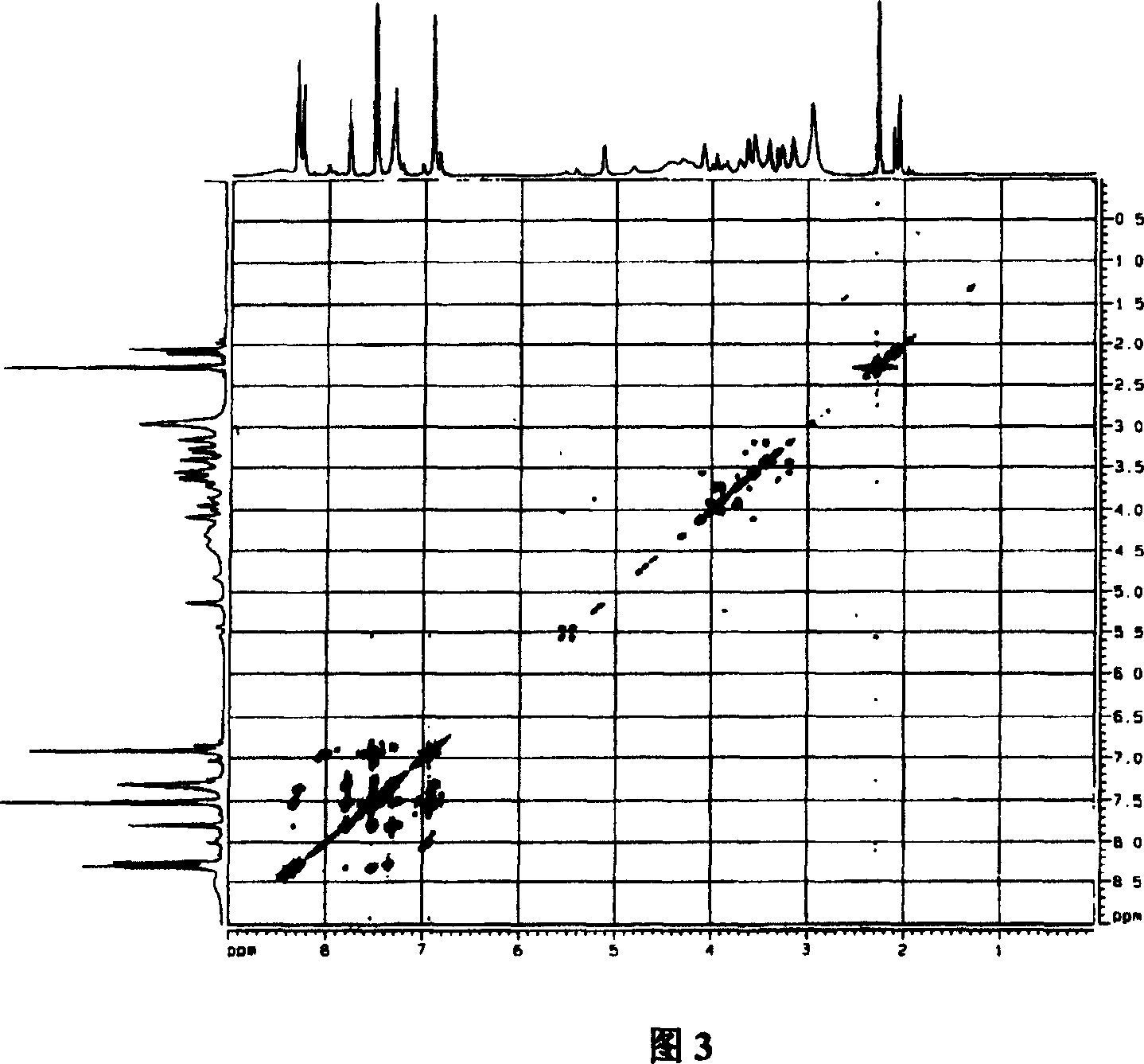
[0044] Mix 9.0g (about 0.05mol) of aspirin with 7ml (about 0.084mol) of thionyl chloride, add 4 drops of pyridine, slowly heat up the oil bath to 75°C within 50min, react at constant temperature for 2h, and evaporate the liquid under reduced pressure , add 6ml of anhydrous tetrahydrofuran, and keep airtight until use. Dissolve 4.2g of puerarin (about 0.01mol) in an appropriate amount of anhydrous tetrahydrofuran, add 5g of anhydrous sodium carbonate, and stir at room temperature for 10 minutes. 5.5 g (about 0.026 mol) of acetylsalicyloyl chloride was slowly added dropwise under stirring in an ice bath, and reacted at room temperature for 1 h, then refluxed for 2 h, filtered, and evaporated to remove the solvent under reduced pressure. The solid was subjected to conventional silica gel column chromatography, chloroform-methanol system gradient elution, silica gel thin layer chromatography (CHCl 3 : MeOH=8:2, 1% FeCl 3 -K 3 [Fe(CN) 6 ] (1:1) solution color development) detec...
Embodiment 2
[0048] Mix 9.0g (about 0.05mol) of aspirin with 5ml of thionyl chloride, add 4 drops of pyridine, slowly heat up the oil bath to 60°C within 50min, react at constant temperature for 2h, evaporate the liquid under reduced pressure, add 6ml of anhydrous Tetrahydrofuran, sealed and stored for later use. Dissolve 10.0 g of puerarin (about 0.025 mol) in an appropriate amount of anhydrous tetrahydrofuran, add 5 g of anhydrous sodium carbonate, and stir at room temperature for 10 min. Slowly add 5.5 g (about 0.026 mol) of acetylsalicyloyl chloride dropwise under ice-bath stirring condition, react at room temperature for 2 hours, reflux for 2 hours, filter and evaporate the solvent under reduced pressure. The solid was subjected to conventional silica gel column chromatography, chloroform-methanol system gradient elution, silica gel thin layer chromatography (CHCl 3 : MeOH=8:2, 1% FeCl 3 -K 3 [Fe(CN) 6 ] (1:1) solution color development) detects, collects and merges R f =0.65-0.8...
Embodiment 3
[0052] Mix 9g (about 0.05mol) of aspirin with 8ml of thionyl chloride, add 4 drops of pyridine, slowly heat up the oil bath to 80°C within 50min, react at constant temperature for 2h, evaporate the liquid under reduced pressure, and add 6ml of anhydrous tetrahydrofuran , sealed and stored for later use. Dissolve 6.2g of puerarin (about 0.015mol) in an appropriate amount of anhydrous tetrahydrofuran, add 5g of anhydrous sodium carbonate, and stir at room temperature for 10 minutes. 5.5 g (about 0.026 mol) of acetylsalicyloyl chloride was slowly added dropwise under stirring in an ice bath, and reacted at room temperature for 1 h, then refluxed for 3 h, filtered, and evaporated to remove the solvent under reduced pressure. The solid was subjected to conventional silica gel column chromatography, chloroform-methanol system gradient elution, silica gel thin layer chromatography (CHCl 3 : MeOH=8:2, 1% FeCl 3 -K 3 [Fe(CN) 6 ] (1:1) solution color development) detects, collects a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com